Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Jonas Amstrup Funder1*, Anne Kraushaar Martensen1,2 and Tommy Bechsgaard3
Received: March 06, 2024; Published: April 05, 2024
*Corresponding author: Jonas Amstrup Funder, Department of Clinical Medicine, Aarhus University, A1001 Aarhus University Hospital, Palle Juul-Jensens Boulevard 99, DK-8200 Aarhus N, Denmark
DOI: 10.26717/BJSTR.2024.56.008781
After abdominal surgery of the gastrointestinal tract, various lengths of postoperative paralytic ileus follow. Prolonged paralysis results in increased morbidity as well as mortality and with no effective treatment options. This study is the first contemporary study investigating gastrointestinal electrical pacing as a novel treatment option for postoperative bowel paralysis. The aim of the study was to define optimal pace settings.
Materials & Methods: Eleven pigs were used for the study. They underwent open abdominal surgery for two hours. Afterwards, pace wires were paced on the serosal surface at five locations in the gastrointestinal tract: stomach, duodenum, jejunum, caecum and rectum. Different pace settings regarding amplitude, pulse width and pulse rate were tested at each location for two minutes, observing for evoked local muscle contractions of the gastrointestinal tract.
Results: A total of 540 measurements were successfully performed. At all pace locations contractions of the gastrointestinal tract postoperatively could be initiated using electrical pacing. Optimal pace settings were defined as the highest rate of muscle contractions following stimulation and were derived for each location as follows: stomach (20V, 500μs, 120Hz), duodenum (10V, 50μs, 25Hz) Small bowel (8V, 50μs, 25Hz), caecum (10V, 200μs, 10/50Hz), and rectum (10V, 200μs, 50Hz).
Conclusions: In this feasibility study we were able to initiate local muscle contractions of the gastrointestinal tract immediately postoperative using an external pacemaker. We, furthermore, determined optimal pace settings for pacing the gastrointestinal tract. These results may serve as the first step towards development of a new treatment option for patients with postoperative paralysis.
Abdominal surgery results in paralysis of the gastrointestinal tract which can last from minutes to several days depending on the surgical trauma [1-4]. Especially major open abdominal surgery is prone to induce gastrointestinal paralysis and prolonged paralysis for more than 5 days can occur in up to 54% of patients undergoing open surgery for advanced abdominal cancer [1-3,5-8]. Prolonged paralysis results in a range of symptoms including abdominal distension, nausea, inability to tolerate enteral nutrition, and non-passage of flatus or stools. This is, furthermore, associated with increased morbidity, prolonged hospital stays and even increased mortality [9,10-12]. Unfortunately, treatment options are sparse and are essentially limited to naso-gastric suction, laxatives, and patience [3,9]. So far, it has not been possible to initiate gastrointestinal motility immediately postoperative. In heart surgery, postoperative dysrhythmias and bradycardias are treated by pacing through temporary pace electrodes placed during the operation [13]. The neuroelectrical anatomy of the heart and gastrointestinal tract, however, is in many ways build the same way [14,15]. Pacemaker centers in the heart - the sinus and sinoatrial node - equals in many ways network of Cajal cells, which lie in the myenteric region between the circular and longitudinal muscle layer and initiate electrical impulses commencing slow wave gastrointestinal contractions. These Cajal networks are located at several regions of the gastrointestinal tract. Other cells of Cajal are also distributed intramuscular along the muscle bundles of the gastrointestinal tract, and - like the Purkinje fibers in the heart-these cells make up a fast track conducting network which enables coordinated muscle contractions [11,16].
In spite of these similarities, pacing the gastrointestinal tract is only performed in a few selected diseases. Especially in diabetic gastroparesis and for faecal incontinentia different pacing methods are well-established treatment options [10,11,16]. Good results from gastrointestinal pacing have also been shown in colonic inertia using colonic pacing [12,17]. However, whether pacing can be used in gastrointestinal surgery to treat postoperative dysrythmia and paralysis, the same way it is used in heart surgery, is unclear. No contemporary research has been made in this area. In this feasibility study our objective is to initiate gastrointestinal contractions immediately postoperative using an external pacemaker as a novel treatment option of postoperative paralysis. Furthermore, we sought to assess optimal pace settings and locations of the gastrointestinal tract in a postoperative setting.
Study Design
The study was conducted as an acute porcine observational study. The experimental animals were anesthetized and submitted for open abdominal surgery for two hours. Immediately postoperative, experiments were conducted investigating the effect of postoperative pacing of the gastrointestinal tract at the following preselected locations: The stomach, the duodenum, the jejunum, the caecum, and the rectum. The effect of the following pacemaker settings was tested at each location: Amplitude, pulse width, and pulse rate.
Experimental Animals
The study comprised 11 Danish Landrace/Yorkshire pigs, with a bodyweight of 60 kg each. Following the experiments, the pigs were euthanized, using an intravenous injection of pentobarbital 400 mg/ml (0,1 ml/kg), during continued anesthesia. The sample size was determined to accomplish the required combinations of pacemaker settings. The primary outcome was occurrence of an evoked gastrointestinal muscle contraction at the paced location.
Experimental Procedure - Anesthesia
The pigs were initially sedated with an intramuscular injection of 0.5 mg/kg midazolam (Dormicum®; Roche) at the research farm. Upon arrival at the laboratory, the pigs were anesthetized with an intravenous injection of 0.5 mg/kg midazolam and 6 mg/kg ketamine (Ketalar®; Warner Lambert) through an ear vein. The animals were intubated and placed on continuous anesthesia with sevoflurane aiming at a mean alveolar concentration of 2-2.7% and with 10μg/kg/h fentanyl (Haldid®; Janssen-Lambert) for pain relief. Each animal was monitored continuously for blood pressure, urine production and electrocardiogram during the entire experiment, with intermittent assessment of arterial blood gas values [18].
Experimental Procedure - Surgery
All experimental animals were subjected to two hours of surgery. We began with a large midline laparotomy from the xiphoid to the os pubis. Immediately after laparotomy the neck was incised on the left side and the neck vessels isolated. The internal carotid artery was cannulated in order to monitor blood pressure. The external jugular vein was cannulated in order to secure a safe venous access. After neck vessel cannulation the animals were subjected to abdominal surgery during the remaining operation time with the following procedures: Cholecystectomy (n=8), full mobilization of the spiral colon (n=9), segmental resection of left lateral liver lobe (n=4), non-anatomical liver wedge resection (n=3), Parietal abdominal wall and retroperitoneal peritonectomy (n=5), mobilization of the caval vein at liver level (n=2), resection of the distal pancreas (n=2), unilateral nephrectomy (n=2), splenectomy (n=4), subtotal hysterectomy (n=4), resection of gastro-colic ligament including the gastro-epiploic artery (n=2), extraperitoneal mobilization of the bladder (n=2), Mattox maneuver (n=1), or Pringles maneuver (n=9). No bowel or stomach resection was performed, thus, keeping the gastrointestinal neural connections intact. The vagal nerve was also not damaged.
Experimental Procedure – Pacing
After two hours of surgery pace wires were sutured on to the surface of the gastro-intestinal tract at the following locations: The stomach, 2/3 distal on the greater curvature; the duodenum, between first and second part; the small bowel, approximately 20 cm from the duodenum; the caecum; and the rectum, just below the recto-sigmoid junction. At each pace site two pace wires were attached one centimeter apart. Following a strict schedule multiple pace settings were tested at each location during continued anesthesia and with the abdomen open. A location was paced for two minutes or till an evoked muscle contraction occurred at the pace site. This was evaluated visually by the investigator. We used an external pacemaker, Test Stimulator 3625 (Medtronic Inc., MN), for testing the following amplitudes: 0.5V, 5V, 8V, and 10V volts. We used another external pacemaker, DISA Stimulater Unit type 14 E 11 (DISA, Denmark), for testing amplitude levels, 15V and 20V. We, furthermore, tested the three different pulse widths: 50μs, 200μs and 450/500μs (depending on pacemaker, data were pooled) as well as six different pulse rates: 5pps, 10pps, 25pps, 50pps, 80pps, and 100/120pps (depending on pacemaker, data were pooled). For each of the gastrointestinal locations (stomach, duodenum, jejunum, caecum and rectum), tests were performed for all amplitude levels (Volt) and combined with all frequencies (Hz). All frequencies were, furthermore, combined with each pulse width (μs) summing up to a total of 540 tests (See Figure 1). The pace current was calculated at selected pace site by measuring the voltage drop of a 10 Ohm series resistor and applying ohms law.
Ethical Considerations
The experiments were conducted according to the guidelines and with approval from the Danish Inspectorate of Animal Experimentation under the Danish Ministry of Justice.
Statistics
Data are presented as actual numbers of positive responses and in percent. Data are compared using logistic regression with clustering allowing for intragroup correlation. A p-value <0.05 was defined as a statistically significant difference. Data were analyzed using Stata IC 15.1 statistical software (StataCorp LLC, Texas, USA).
Amplitude
Amplitude levels were tested in the range of 0.5-20V. For the different pace locations, the optimal amplitude levels were as follows. The stomach reached a contraction rate of 72% at 20V (P=0.02). The duodenum had the highest contraction rate at 8-15V (78-94%). Maximum contraction rate was at 10V (P<0.001). The jejunum had the highest contraction rate at 8-10V (94-100%). The maximum contraction rate was at 8V (P=0.005). The caecum generally had a high contraction rate especially at 10 and 20V (94%) (P<0.001). The rectum had the highest contraction rate at 8-20V (89-100%). The maximum contraction rate was at 10V (P=0.001). Generally, the low amplitude levels of 0.5 and 5V exhibited the lowest contraction rate, however the caecum and rectum still responded acceptably at this level. See Table 1 for the respective electrical currents as well as Table 2 and Figure 2 for further details on amplitude.
Table 2: Number of contractions and odd ratios for different amplitude levels. Data is given as actual number (percent) and as odds ratio (OR) with 95% confidence interval. A hyphen predicts perfect success or perfect failure. N=18 measurements for each amplitude level at each location in gastrointestinal tract.

Pulse Width
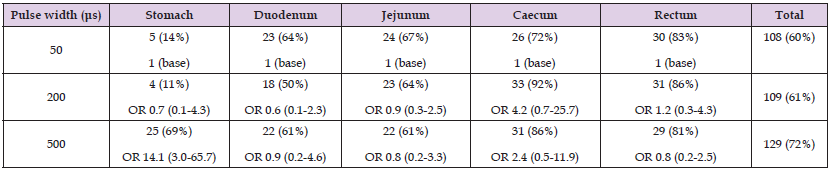
Pulse width levels were tested in the range of 50-500μs. For the different pace locations, the optimal pulse width levels were as follows: The stomach reached 69% contraction rate at 500μs (P=0.1). At lower pulse width levels, the contraction rate was considerably reduced (11-14%). The duodenum showed contraction rates between 50% and 64%. The maximum number of contractions was seen at a pulse width of 50μs (P=0.4). Likewise, the jejunum reached acceptable contraction rates at all pulse width levels (61-67%). The maximum number of contractions was seen at a pulse width of 50μs (P=0.4). This was even higher for both the caecum and rectum, which reached a high contraction rate for all pulse width levels of respectively 72-92% and 81-86%. The maximum number of contractions was seen at a pulse width of 200μs for both pace locations (P<0.001 and P=0.03 respectively). See Table 3 and Figure 3 for further details.
Table 3: Number of contractions for different pulse width levels. Data is given as actual number (percent) and as odds ratio (OR) with 95% confidence interval. N=36 measurements for each pulse width level at each location in gastrointestinal tract.
Pulse Rate
Pulse rate levels were tested in the range of 5-120Hz. For the different pace locations, the optimal pulse rate levels were as follows: The stomach contraction rate peaked at 39% at 120Hz (P=0.4). At lower pulse rate levels, the stomach did not respond well (22-28%). The duodenum exhibited the highest contraction rate at 25Hz (78%) (P=0.06). Generally, the duodenum had contraction rate levels above 50%. The jejunum also exhibited the highest contraction rate at 25Hz (72%) (P=0.2). Generally, the jejunum had contraction rate levels above 56%. The caecum generally had high contraction rates ranging between 67-89%. The highest contraction rate was seen at 10, 50 and 80Hz (P=0.006). This was even better for the rectum, which reached high contraction rates for all pulse range levels of 78-94%. The highest number of contractions was seen at 50Hz (P=0.03). See Table 4 and Figure 4 for further details.
Table 4: Number of contractions for different pulse rates. n=number of measurements per pulse rate value. Data is given as actual number (percent) and as odds ratio with 95% confidence interval. N=18 measurements for each pulse rate level at each location in gastrointestinal tract.

In this study we have demonstrated the possibility of pacing the gastrointestinal tract in the postoperative setting. We, furthermore, identified both optimal settings of the pacemaker as well as possible pace locations.
From the beginning of heart surgery temporary pace electrodes were used to treat postoperative bradycardias. Inspired by this, Bilgutay et al. developed a gastrointestinal pacemaker in 1963 in order to treat postoperative paralysis. Although heart surgeons implanted temporary pacemakers directly in the muscle of the heart Bilgutay et al. chose to pace the gastrointestinal tract using nasogastric tube electrodes as this method is less invasive [7]. Initial experiments seemed promising, but further clinical studies conducted in the same period did not support the concept why this line of research was abandoned until now [9-14]. These clinical studies, however, were small - comprising 17-48 patients. Furthermore, the method of intraluminal ventricular pacing implies great risk of connection failure as electrodes are placed blindly inside the stomach. In a clinical setting with paralysis the stomach must be filled and dilated and as the electrodes are not attached at the stomach wall a secure connection must be difficult to obtain [9]. In order to secure a proper connection between the pace wires and the gastrointestinal tract another approach could, therefore, be to implant temporary pacemakers directly on the wall of the gastrointestinal tract [19]. This was the approach we used in the present study with promising results.
We were, thus, able to initiate local contractions in the gastrointestinal tract in a postoperative setting. Pacing the GI-tract is a technique used in other diseases as well. Especially in gastroparesis implantation of a permanent pacemaker is a well-established method and can also be performed as a minimally invasive procedure [20,21]. Also, sacral nerve stimulation for anal incontinence is an integrated method in clinical practice and has proven excellent results. The method does not entail direct stimulation of the bowel or sphincter but uses indirect stimulation through the sacral nerve [10]. The method of sacral nerve stimulation is also being tested for irritable bowel syndrome as well [11]. Shafik et al. also presented a method to treat constipation due to colonic inertia with promising results. They used a similar method as us with direct stimulation of the colon. They, however, used an endoscopic method to place the electrodes within the mucosa and muscularis [12,17]. Interstitial cells of Cajal have been described for the first time by Spanish neuroanatomist Santiago Ramon y Cajal (1852–1934) in 1889. They are pacemaker cells and are found throughout the gastrointestinal tract from the esophagus to the internal anal sphincter. They are organized in a network placed both intermuscular and intramuscular [14,22]. They initiate slow wave impulses starting at the antrum and major curvature of the ventricle and then distribute the impulses along the small bowel. In the colon the initiation of the electrical impulses is still debated.
However, experimental studies have identified at least four potential pacemaker sites located at the cecal pole, the caecocolonic junction, the mid-transverse colon, and the colosigmoid junction. At these sites groups of myenteric Cajal cells are located between the circular and longitudinal muscle layer [12,17]. Our choice of pacing location was defined from this knowledge and from an intention to include representative sites from the entire gastrointestinal tract. The stomach required an excitation level of 10-20V before a minimum number of contractions could be detected. This indicates that direct muscle pacing is the most effective approach for the stomach as opposed to nerve stimulation. As we chose to place our electrodes on the wall instead of into the wall of the gastrointestinal tract, higher amplitudes could be needed to penetrate the thick wall of the stomach. This might explain the difference when comparing clinical pace settings in treatment of gastric paresis where a lower amplitude is generally needed. In the clinical treatment of gastropareses electrodes are placed within the layer of muscularis propria [20,21]. Furthermore, clinical pacemakers typically do not reach higher levels than 10.5V. This will practically make clinical trials with higher amplitude levels impossible at the moment. Whether these high amplitude levels also entail a clinical side-effect such as stimulation of the muscles of the abdominal wall is also unanswered as we performed our studies at open abdomen conditions. In the other locations of the gastrointestinal tract contractions were possible for most of the amplitude levels except from 20V at the jejunum, where only few contractions were seen.
The efficacy for different pulse widths as well as pulse rates did not differ much in our study indication that both nerve and muscle may be stimulated postoperatively with the same result. According to the literature, a number of different settings have been utilized in different experiments at different segments of the gastrointestinal tract with positive outcome [23-25]. The exact pulse rate and width may, thus, not be that important for postoperative gastrointestinal pacing, as long as it is within the range of the current study. Only for the stomach we found a noticeably better effect in the high pulse rate as well as the high pulse width compared with the lower settings. This effect was also different from the settings used clinically for gastric pacing regarding the pulse rate [26]. Again, the extra-serosal placement of the electrodes along with the thick wall of the stomach may contribute to this result. However, when transforming our experiment to clinical use one must also bear in mind which location would be suitable clinically to place potential pace wires for postoperative pacing. Off course the stomach could be obvious as normal slow waves starts here. The stomach is also the only location that is used for direct muscle pacing in a clinical setting in the treatment of gastroparesis.
The fact that the stomach is adjacent to the abdominal wall diminishes the amount of wire inside the abdomen considerably. The wire inside the abdominal cavity entails a risk of obstructing ileus. The duodenum is not that obvious to use in a clinical setting as most of the duodenum is located retroperitoneally in humans. Likewise, the small bowel is not the best clinical pace site as the small bowel on the other hand is very mobile. Furthermore, the small bowel is not the starting point of the slow waves and are. Additionally, the small bowel is normally the first bowel segment to initiate contractions after surgery why pacing the small bowel seems less important. The colon and rectum is normally the last part of the gastrointestinal tract to initiate contractions after surgery and could, consequently, also be a good location to place pace electrodes [9]. The exact placement would also depend on what kind of surgery and bowel resection is performed (Figure 5).
Study Limitations
This was an acute study with many measurements performed on the same animal. Sometimes migrating motor complexes occurred and further measurements would have to be put on hold till these contractions ceased. Most often, however, we would register segmental contractions in the near vicinity of the pace electrodes only, and then we would move on to the next location. However, it seems likely that if we kept pacing more than the 2 minutes, the local segmental contractions would develop into migrating motor complexes. This must be verified in a chronic animal model before human studies can be carried out. Another limitation of our study is the porcine nature of our experiment. Ultimately, these results must be reproduced in a human model before expanding the results into general clinical practice. However, further animal studies are needed before initiating human studies.
In conclusion, we were able to initiate local gastrointestinal contractions immediately postoperative using an external pacemaker. We, furthermore, determined optimal pace settings for pacing the gastrointestinal tract. These results may serve as the first step towards a possible treatment option for postoperative paralysis.
The authors would like to thank the staff at the Department of Clinical Medicine, Aarhus University, especially animal technician Kira Graahede. We would, furthermore, like to thank the Biostatistical Advisory Service at Aarhus University for statistical assistance. The study could not have been performed without financial support from Arvid Nilssons Fond and Helga and Peter Kornings Fond to which we are thankful.
Arvid Nilssons Fond and Helga and Peter Kornings Fond.
• Jonas Amstrup Funder: planning and conducting the study, collecting, and interpreting data, and drafting the manuscript.
• Anne Kraushaar Martensen: planning and conducting the study, collecting, and interpreting data, and drafting the manuscript.
• Tommy Bechsgaard: conducting the study, collecting, and interpreting data, and drafting the manuscript.
None.


