Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Adewale Lawrence*
Received: March 13, 2024; Published: April 02, 2024
*Corresponding author: Adewale Lawrence, Department of Medicine, Faculty of Medicine, Universitatea de MedicinășiFarmacie “Grigore T. Popa” Iași, Romania
DOI: 10.26717/BJSTR.2024.55.008771
Background: Nosocomial infections, acquired during hospital stays, pose a global health concern, particularly in developing countries. Variances in epidemiology and etiology exist among nations and even within hospitals. Multi-antibiotic-resistant microorganisms, easily transmitted, cause pneumonia, bloodstream, urinary tract, and surgical wound infections in Nigeria. Antimicrobial resistance amplifies the risks of illness, death, and healthcare expenses.
Objective: This study aims to identify the root causes of nosocomial infections in Nigeria, focusing on Ogun State University Teaching Hospital, Sagamu.
Methods: Data collected from 854 patients admitted to surgical wards or SICU revealed a 9.1% prevalence of nosocomial infections. Among 215 selected patients, 38 (49.4%) had surgical site infections, 23 (29.8%) had urinary tract infections, and 16 (20.8%) had bloodstream infections. Gram-positive and Gram-negative microorganisms exhibited 23.4% and 72.6% prevalence, respectively. E. coli, S. aureus, and Klebsiella species were predominant, with 9.1% of patients having multiple bacterial agents.
Results: Complete resistance was observed in Gram-positive bacteria to penicillin, ampicillin, tetracycline, chloramphenicol, and trimethoprim-sulphamethoxazole. Gram-negative bacteria exhibited 100% resistance to amoxicillin, tetracycline, and trimethoprim-sulphamethoxazole. Empirical treatment may be ineffective due to widespread medication resistance, emphasizing the need for culture-based sensitivity in treatment decisions.
Conclusion: Ampicillin, Tetracycline, Trimethoprim-sulphamethoxazole, and Chloramphenicol were all completely resistant to the gram-positive bacteria that were isolated from nosocomial infections. Furthermore, all isolates of Gram-negative bacteria were completely resistant to trimethoprim-sulphamethoxazole, tetracycline, and amoxicillin. Nosocomial infections may not respond well to empirical treatment because of the high prevalence of medication resistance in the microorganisms. As a result, the basis for treatment ought to be sensitivity and culture.
Keywords: Nosocomial Infections; Epidemiology; Etiology; Multi-Antibiotic Resistance; Healthcare-Associated Infections
Abbreviations: SSIs : Surgical Site Infections; NIS: Nosocomial Infection; UTI: Urinary Tract Infection; SICU:Surgical Intensive Care Unit
Nosocomial infections (Nis) are defined as infections that a patient gets during their stay in a hospital and are either absent or not in the development phase at the time of admission to the hospital. Depending on how long the infection takes to nurture, the signs and symptoms of the illness may appear during the hospital stay or after discharge. It is commonly described as an infection discovered at least two to three days following the patient's admission to a medical facility. Hospital-acquired infections have the potential to worsen pre-existing conditions, cause distress and anxiety, and may even be deadly [1]. Significant morbidity, death, and increased financial burden are the outcomes of these illnesses. Nosocomial infections are significant public health issues in both industrialized and underdeveloped nations [2]. Numerous illnesses are linked to microorganisms that are multi-antibiotic resistant and easily transmitted by staff hands [3]. Over 1.4 million patients globally currently have infectious issues that they contracted while in the hospital. Compared to other hospital wards, nosocomial infection and death are more common in intensive care units. The risk is five to ten times higher for patients in the intensive care unit. Hospital patients' immune systems are failing to prevent nosocomial infections more and more [4].
Between 25 and 50 percent of nosocomial infections result from the interaction of intrusive equipment with the patient's microbiota. Nowadays, the majority of infections obtained in hospitals are brought on by ordinary household microorganisms (e.g., enterococci, coagulase-negative staphylococci, Staphylococcus aureus, Enterobacteriaceae) that either do not cause illness in people or cause it less severely than in hospital patients [5]. Antimicrobial resistance has been more common over the last few decades, and it has been alarmingly linked to major infectious illnesses [6]. While nosocomial infections can occur in a variety of locations, the most prevalent ones include lower respiratory tract infections, surgical site infections, urinary tract infections, and bloodstream infections. Because patients with Surgical Site Infections (SSIs) typically stay in the hospital longer, are more likely to die, and are readmitted more frequently, SSIs are the most prevalent source of nosocomial infections, which have a significant impact on morbidity and mortality [7-9]. S aureus, P. aeruginosa, E coli, K pneumoniae, Enterobacter spp., Enterococcus spp., CoNS, and Viribans streptococci are the most prevalent bacterial pathogens [10,11].
A Urinary Tract Infection (UTI) is characterized by the growth of a single pathogen in a correctly collected mid-stream urine specimen that has more than 105 colony-forming units/ml [12], According to (Kehinde, et al. [13]) UTIs can result in significant morbidity for patients who have an indwelling urethral catheter. According to (Hsueh, et al. [11]), the most prevalent bacterial pathogens include P aeruginosa, E. coli, S. aureus, K. pneumoniae, Enterobacter spp., Enterococcus spp., Proteus spp., and Citrobacter spp. Approximately 10% to 30% of pediatric hospital-acquired illnesses are caused by bloodstream infections, which are among the most prevalent infections [14]. S. aureus, CoNS, P. aeruginosa, K. pneumoniae, E. coli, Enterobacter spp., Enterococcus spp., and Acinetobacter spp. are the predominant bacterial pathogens in cases of BSI. Ventilator-related pneumonia, which is nosocomial bacterial pneumonia that develops after two days of mechanical ventilation, is the most frequent nosocomial infection encountered in the critical care unit [15]. Staphylococcus aureus, Pseudomonas aeruginosa, and Enterobacteriaceae are the most common organisms that cause infections. The makeup of patients in an intensive care unit, the duration of hospitalization, and the use of previous antimicrobial medication all influence the etiologic agents [16].
Hospital-acquired infections are often the result of breakdowns in the body's defensive mechanisms caused by invasive surgeries and antibiotic usage. Iatrogenic, organizational, or patient-related risk factors are possible. Specialized laboratory tests used for diagnosis include radiography for pneumonia and blood cultures for bloodstream infections. Concern over antibiotic resistance is growing, especially about microorganisms that cause nosocomial infections. Although the goal of prevention efforts is to stop the emergence of resistant organisms and lessen antibiotic resistance, many infections cannot be prevented because of aging, chronic illnesses, and suppression of the immune system [17,18]. Nosocomial infections, prevalent in underdeveloped nations, lack a dedicated control program in our country, as highlighted by studies at Ogun State University Teaching Hospital, Sagamu. The research aims to assess infection frequency, drug susceptibility patterns, and associated risk factors, recognizing the global impact of antimicrobial resistance.
A cross-sectional study was carried outAt Ogun State University Teaching Hospital, Sagamu in Sagamu, Ogun State, Nigeria, using information gathered between July 2010 and September of next year. Patients with suspected nosocomial infections who were fifteen years of age or older were the subject of this investigation, which was conducted in the hospital's surgical wards and Surgical Intensive Care Unit (SICU). The research area was Ogun State University Teaching Hospital, Sagamu, a well-known institution in the area with five hundred and sixty beds that were conveniently positioned and provided local emergency services. The dependent variables included patterns of antibiotic susceptibility, septicemia, surgical wound infection, and urinary tract infection (UTI). Age, sex, the placement of a urinary catheter, surgery, mechanical breathing, intravascular catheter, use of antibiotics, and length of stay were among the independent variables taken into account. Physicians conducted a comprehensive clinical assessment to identify potential risk factors and rule out community-acquired infections as part of a convenient sampling approach. Following WHO guidelines, the sample size (n) was established using the highest recorded nosocomial infection prevalence (16.4%) at Sagamu Hospital, with a 0.05 margin of error and a 95% confidence interval (Zα/2). Patients meeting the defined criteria (i.e., developing at least two to three days after admission to surgical wards and ICU) for UTI, primary bacteremia, or surgical wound infection were eligible for inclusion in the study. Patients with infections obtained in the community were not included in the study. This choice was made to concentrate on nosocomial infections and guarantee a steady period of infection growth after hospitalization.
A questionnaire that had been previously created and evaluated was used to gather data on sociodemographic characteristics and related risk factors. Sample collection: Based on clinical observations, specimens were taken from patients suspected of acquiring nosocomial infections who were admitted to the surgical wards and SICU. Standard operating procedures were followed in the collection and analysis of the specimens. Blood collection and processing: When a bloodstream infection was suspected, adult patients with fever or chills had their blood samples taken aseptically. Ten milliliters of venous blood were drawn, and the blood was promptly inoculated into a tube that had soup that contained thioglycollate. For ten days, blood was incubated aerobically at 37 °C, and turbidity was measured as a sign of growth (Annex 1). Urine specimens: A sterilized container was used to collect a urine sample for bacteriological analysis, and both before and after the catheter was used, the sample was cultured. This procedure was done utilizing the midstream approach. Urine cultures with colony counts greater than 105 CFU/ml of urine following catheterization and urine samples without substantial growth (< 104 CFU/ml of urine) before catheter placement were deemed suggestive of severe infection (Annex 2). Swabs from wound infections: Infections from wounds arise from illnesses, injuries, or surgical procedures that disrupt the skin's outer layer. To separate the causing agents, material from infected wounds was collected aseptically (Annex 3).
Culture and Gram staining: While urine, swabs, and body fluid specimens were inoculated on blood agar and MacConkey agar, blood specimens were inoculated in thioglycollate broth (Oxoid, Ltd.). The MacConkey agar plates and blood were incubated at 37 °C in an aerobic atmosphere for a duration of one to two days. Thioglycollate soup was incubated at 37 °C in an aerobic environment for a maximum of two weeks. Every day for up to fourteen days during that period, the area was checked for any visible signs of bacterial development. For all positive specimens, subcultures were subsequently carried out on Blood, Chocolate, and MacConkey agar plates. According to Cheesbrough [19], positive cultures were recognized by their unique looks on the corresponding media, the Gram-staining reaction (Annex 4), and the pattern of biochemical reactions. Biochemical tests: UtilisingAPI 20E identification kits (Biomerieux, France), members of the Enterobacteriaceae family were identified by oxidase, carbohydrate utilization, motility testing, urease testing, citrate utilization, indole synthesis, and other assays. Coagulase, DNase, catalase, bacitracin, and optochin susceptibility tests were utilized for Gram-positive bacteria.
Antimicrobial susceptibility testing: All isolates underwent antimicrobial susceptibility testing using the disk diffusion method, following (Bauer, et al. [20]) and the National Committee for Clinical Laboratory Standards [21] Three to five colonies of pure-cultured bacteria were collected, transferred, and gently mixed with five milliliters of nutrient broth to create a homogenous suspension. The suspension was then incubated at 37 degrees Celsius until its turbidity was corrected to the 0.5 McFarland standard. The surplus suspension was eliminated by gently rotating a sterile cotton swab against the tube's surface. Next, using a swab, the bacteria were uniformly distributed throughout the surface of Mueller-Hinton agar and Mueller-Hinton agar enriched with 5% sheep blood, which was specifically utilized for S. pneumoniae (Oxoid). Following three to five minutes of drying at room temperature for the inoculation plates, a set of sixteen antibiotic discs (Oxoid) was placed on the surface of a Muller-Hinton plate. The following concentrations of medications were used in disc diffusion testing: After that, the plates were incubated for one to two days at 37 °C.
Using a caliper, the diameters of the zone of inhibition surrounding the disc were measured to the closest millimeter. The isolates were then categorized as sensitive, moderate, or resistant using the NCCLs' standard table (NCCLs, 2006) [21]. According to Annex 5, resistance levels are classified as high, intermediate, and low when the percentages are, respectively, >80%, 60-80%, and < 60%. The EHNRI laboratory stock was utilized as a quality control measure for antimicrobial susceptibility testing and culture throughout the investigation.
Data Entry and Analysis
Software called SPSS12.0 was used for data entry and analysis. The Chi-square test was used to compare the results. A difference that was deemed statistically significant was indicated with a p-value of less than 0.05. The total number of positive instances among the patients that were investigated was used to compute the prevalence rate.
Ethical Considerations
The Department of Hygiene – Environmental Health of the "Grigore T. Popa" University of Medicine in Iasi, Romania, has validated this graduation thesis. The technique of sampling carried little risk; it was no different than taking a specimen for sensitivity and culture in a regular laboratory. Sterile swabs and disposable syringes with needles were used to stop the spread of HIV and other infectious diseases. Every piece of information that was included in the questionnaire was kept private. Every person who received a nosocomial infection diagnosis gave their informed consent. Patients had the option to leave the study if they were not interested in it. The management of nosocomial infections was contingent upon the findings of antibiotic sensitivity. Before the actual data collection period began, a letter explaining the study's purpose to the hospital's medical director was filed.
The incidence of nosocomial infections was examined using data from 854 patients who were hospitalized at Ogun State University Teaching Hospital, Sagamu in Ogun State's surgical ward and intensive care unit between July 2010 and September of next year. Upon admission, a thorough clinical examination was conducted to rule out infections acquired in the community and identify any potential risk factors. A total of two hundred and fifteen (25.17%) patients were chosen from the surgical wards (n = 161) and SICU (n = 54) of Ogun State University Teaching Hospital, Sagamu based on their clinical background. There were two hundred and fifteen patients total; eighty-five (39.5%) were female and one hundred and thirty (60.5%) were male. The male-to-female ratio was 1.5:1. The age was 38.02 (+14.82) years, with a range of seventeen to seventy-nine years. With a range of 3 to 66 days, the average hospital stay from the date of admission until sample collection was 16.72 days. Table 1 displays the age and sex distribution of the patients who were looked into for nosocomial bacterial infections. From the 215 patients included in the study, various samples were collected, comprising 88 pus swabs from the infection site, 84 urine samples, and 43 blood samples. Wound classification revealed that out of the total cases, 153 wounds were categorized as clean, 42 as clean-contaminated, and 20 as contaminated. Sixty-five percent of the patients received antibiotic prophylaxis before sample collection. Approximately twenty-eight primary reasons (diagnoses) were given for admission in this study; of these, benign prostatic hyperplasia (BPH) accounted for thirty-three (15.3%), car accidents for twenty-five, 11.6%, bullet injuries for twenty-four, 11.2%, head injuries for twenty, 9.3%, urethral stricture for thirteen, 6.0%, esophageal cancer for thirteen 6.6%, intestinal obstruction for twelve, 5.6%, acute appendicitis for ten (4.7%), and other causes for sixty-five (30.3%).
Table 1: Age and sex distribution of 215 patients investigated for bacterial nosocomial infections at Ogun State University Teaching Hospital, Sagamus, Sagamu, Ogun state, Nigeria (July 2010 - September 2011).
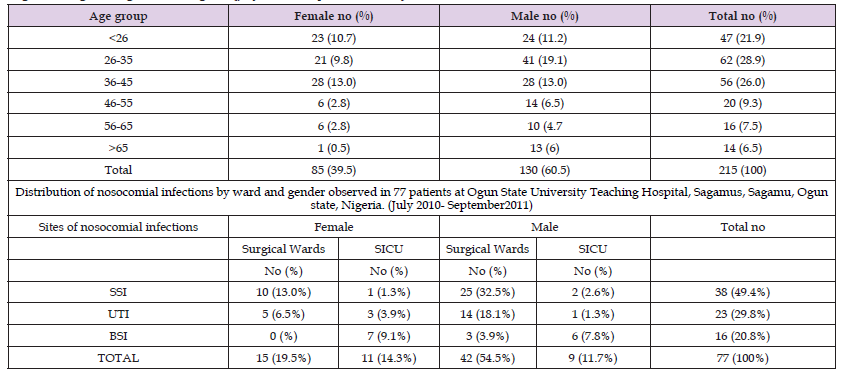
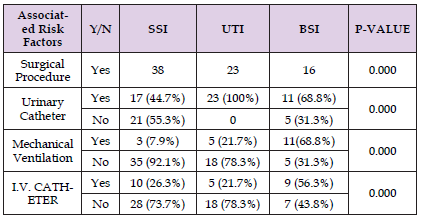
Seventy-seven (9.0%) of the eight hundred fifty-four patients had nosocomial infections. Fifty-five (66.2%) males and 26 (33.8%) females made up the seventy-seven patients. Table 2 explains the nosocomial infection distribution among positive patients. It shows that thirty-eight (49.4%) of the infections were at the surgery site, twenty-three (29.8%) were urinary tract infections, and 16 (20.8%) were bloodstream infections. Eight (10.4%) of the 23 patients with urinary tract infections were female, and fifteen (19.4%) were male. Of sixteen patients with bloodstream infections, seven (9.1%) were female, and nine (11.7%) were male. Of the 38 patients with surgical site infections, eleven (14.3%) were female, and twenty-seven (35.1%) were male. Table 2 illustrates that BSI was most commonly seen in the SICU among other wards. In this investigation, there was a substantial (p < 0.05) correlation found between nosocomial infection and surgical operations, urine catheter insertion, central venous catheter insertion, and mechanical ventilation (Table 2).
Table 2: Nosocomial infections and associated risk factors at 77 patients at Ogun State University Teaching Hospital, Sagamu, Sagamu, Ogun state, Nigeria (July 2010- September2011).
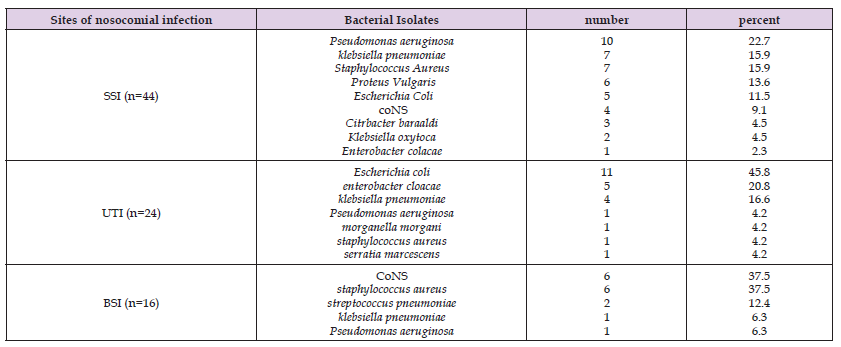
From 77 nosocomial infection cases, a total of 84 bacterial isolates were obtained. 19.0% of the isolates were E. Coli, followed by S. aureus (16.7%), Klebseiella spp. (S. pneumoniae and K. oxytoca) (16.7%), P. aeruginosa (14.3%), coagulase negative staphylococcus (11.9%), P. vulgaris and E. cloaceae (7.1%), S. pneumoniae and Citrobacter spp. (2.4%), and Serratia spp. and Morgenella spp. (1.2%) (Figure 1). segregated from patients hospitalized in the surgical ward and intensive care unit (SICU) of Ogun State University Teaching Hospital, Sagamus, located in Sagamu, Ogun State, Nigeria, between July 2010 and September 2011. In 7/77 (9.1%) of the nosocomial infection cases, more than one bacterial etiologic agent was detected (data not shown). 23/84 (23.4%) and 61/84 (72.6%) of the microorganisms were Gram-positive and negative, respectively (p<0.05). Table 3 explained the distribution of bacterial isolates from patients admitted to the surgical ward and Surgical Intensive Care Unit (SICU) revealed notable patterns in the sites of nosocomial infection. Among the cases of surgical site infection (n = 44), Pseudomonas aeruginosa (22.7%), Klebsiella species (K. pneumoniae and K. oxytoca) (20.4%), and Staphylococcus aureus (15.9%) were the predominant isolates. Urinary tract infections (UTI) (n = 24) were primarily associated with Escherichia coli (45.8%), E. cloacae (20.8%), and K. pneumoniae (16.6%). In cases of bloodstream infections (BSI) (n = 16), Staphylococcus aureus (37.5%), coagulase-negative staphylococci (37.5%), and Streptococcus pneumoniae (12.4%) were the most frequently identified bacterial pathogens.
Table 3: Sites of nosocomial infection and distribution of bacterial isolates from patients who were admitted in surgical ward and SICU at Ogun State University Teaching Hospital, Sagamus, Sagamu, Ogun state, (July 2010- September 2011).
All of the patients who were part of these investigations had received antibiotics, either therapeutically or prophylactically, and seventy-seven of them had cultures that came back positive. Antimicrobial susceptibility testing
a) Gram-Positive Bacteria
The susceptibility patterns of 26 Gram-positive bacteria against 14 antimicrobial medicines that were isolated from nosocomial illnesses are shown in Table 4. Nearly every microbe isolate had multiple drug resistance or resistance to two or more medications. The majority of isolates showed 100% high-level resistance to penicillin, ampicillin, tetracycline, chloramphenicol, and trimethoprim-sulphamethoxazole, and >80% (high level of resistance) to amoxicillin-clavulanic acid, gentamicin, cloxacillin, methicillin, amoxicillin, and doxycyclin. On the other hand, there was very little (<60%) resistance to ciprofloxacin, norfloxacine, and ceftriaxone.
b) Gram-Negative Bacteria
The sensitivity profiles of thirteen antimicrobial medications against 58 Gram-negative bacteria that were isolated from nosocomial illnesses are presented in Table 5. Each isolate showed a full high level of resistance to amoxicillin, tetracycline, trimethoprim-sulphamethoxazole, and ampicillin, as well as >80% (high degree of resistance) to amoxicillin-clavulanic, chloramphenicol, and doxycyclin. Gentamicin was the only drug to show moderate resistance (60–80%). On the other hand, there was very little (<60%) resistance to ceftriaxone, nalidixic acid, nitrofurantoin, norfloxacine, and ciprofloxacin. Similar to Gram-positive bacteria, nearly all discovered Gram-negative bacteria showed multi-drug resistance.
Table 4: Antimicrobial Susceptibility Patterns of Gram-Positive Bacteria Isolated fromNosocomial Infections from patients who were admitted to the surgical ward and SICU atOgun State University Teaching Hospital, Sagamus, Sagamu, Ogun state (July 2010 – September2011)ANTIMICROBIAL AGENTS.
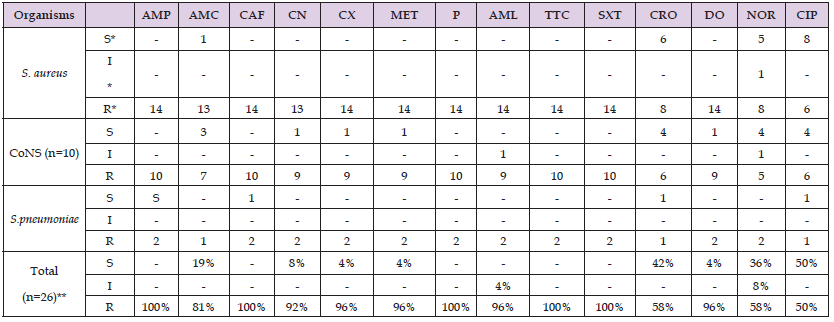
Note: *S= Sensitive *I=Intermediate *R=Resistant ** Expressed in percent. AMP: Ampicillin; AMC: Amoxicillin-Clavulanic acid; CAF: Chloramphenicol; CN: Gentamicin;CX: Cloxacillin; MET: Methicillin; P: Penicillin; AML: Amoxicillin; TTC: Tetracycline; SXT: Trimethoprim-sulphamethoxazole; CRO: Ceftriaxone; DO: Doxycyclin; NOR: Norfloxacine; CIP: Ciprofloxacin.
Table 5: Antimicrobial Susceptibility Patterns of Gram-Negative Bacteria Isolated from Nosocomial Infections from patients who were admitted to the surgical ward and SICU at Sagamu University Hospitals, Sagamu, Ogun state. (July 2010- September2011).
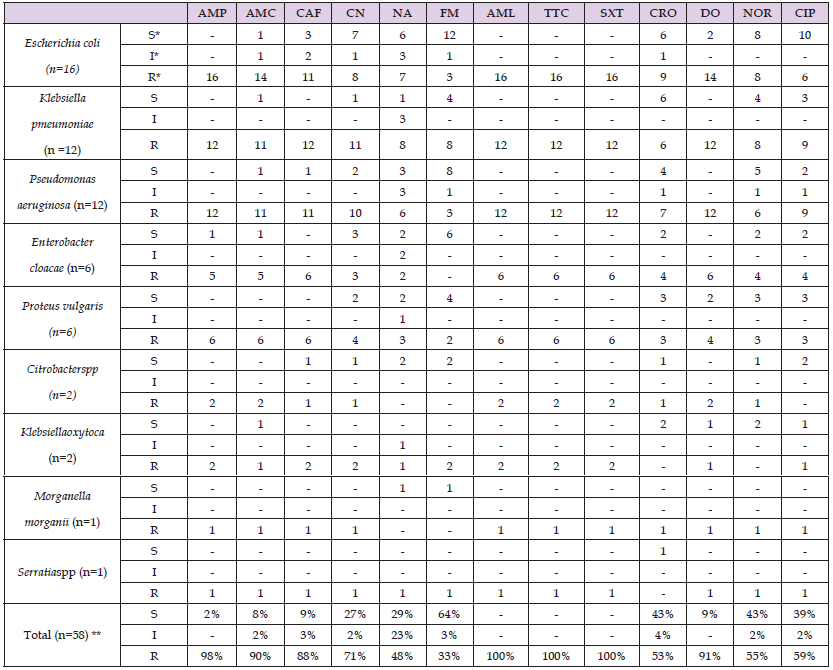
Note: *S= Sensitive *I=Intermediate *R=Resistant ** Expressed in percent.
The incidence of nosocomial infections was examined using data from 854 patients who were hospitalized at Ogun State University Teaching Hospital, Sagamu in Ogun State's surgical ward and intensive care unit between July 2010 and September of next year. Upon admission, a thorough clinical examination was conducted to rule out infections acquired in the community and identify any potential risk factors. Hospitalized individuals are experiencing an increasing number of nosocomial infections (NIs) [22]. Major causes of mortality and disability exist in every country. The World Health Organization has estimated that up to 15% of hospitalized patients have illnesses related to medical treatment [23]. Furthermore, the problem of antimicrobial-resistant bacteria spreading and proliferating in hospitals around the globe persists, especially concerning those microorganisms that cause nosocomial infections in intensive care unit patients [11]. The total nosocomial infection prevalence at Sagamu University institution (9.0%) is lower in this study than it was in the earlier studies conducted in the same institution (16.4%) [24], 17% [25]; and 13% in Kenyan teaching hospitals [26].
Nosocomial infection rates in this hospital have slightly decreased, which could be attributed to following established protocols for decontaminating and cleaning soiled objects and other items, sterilizing and using high-level disinfection techniques, and enhancing safety in operating rooms and other high-risk areas where the majority of serious injuries and infectious agent exposures take place. The layout of the facility and the abundance of medical staff may also be contributing factors to the lower infection rate. However, in contrast to the current study, the prior report included every hospital department. Another explanation might be that a novel approach for selected situations from the emergence was implemented within two days of its emergence during the research period. The brief exposure time before to surgery might be a factor in the surgical wards' low nosocomial infection prevalence rate.
In addition, the present investigation revealed a lower incidence of nosocomial infection than those reported in other nations, such as Tunisia (13%) [27], Kosovo (17.4%) [28], and Morocco (17.8%) [29]. This reduced prevalence might have resulted from the sample analysis method's reliance on bacteriological agents. Since anaerobic bacteria can also cause nosocomial infections, they are excluded from this list. Even though viral and fungal agents have the potential to cause nosocomial infections, they were not evaluated in this investigation since technology and laboratory facilities were not available. However, the majority of the studies conducted in the aforementioned nations also included anaerobic bacteria, fungi, and viruses. Surgical site infections were shown to be the most common nosocomial infection in this investigation. Since every patient has to have surgery, there is a higher chance that they may become infected in the hospital due to germs directly entering their bodies and finding a way into normally sterile areas of their bodies. Healthcare personnel or infected surgical equipment are the two possible sources of this illness. Additionally, extended hospital stays and inadequate wound care increased the risk of surgical wound infections.
Owing to the aforementioned cause, surgical wounds are the most common sites of nosocomial infections. The second infection location in the current investigation was a urinary tract infection. Since catheterization raised the risk of infection, all of the patients with nosocomial UTIs had urinary catheters. Bacteria may exist in or near the urethra, but they are often unable to reach the bladder. An infection can result from microorganisms that a catheter brings into the bladder from the urethra. Compared to other surgical wards, the SICU had a higher prevalence of BSI infections (4.3 times higher) than any other infection location. This is due to the severely sick nature of the SICU patients, as well as the increased risk of colonized bacteria entering the lungs in patients who are unable to cough or gag. Certain breathing techniques can prevent patients from coughing or gaging. Coughing and gag reflexes may also be absent in patients who are drugged or who lose consciousness.
As a result, the microbe that was inhaled develops in the lungs and starts an infection that may spread to the bloodstream.
Furthermore, several interventions that were risk factors for BSI were routinely carried out in this ward, including the use of invasive devices, mechanical ventilation, suction of material from the throat and mouth, the use of medications, and the impact of surgery. It should come as no surprise that the frequent treatments performed in hospitals—mechanical ventilation, urine catheterization, surgery, and central venous line insertion—are the ones that cause nosocomial infections. The surgical wards are where such invasive operations are frequently performed, and these wards are turning into reservoirs for germs that are resistant to many drugs. According to tab IV of the current study, there is a strong correlation between these therapies and nosocomial infections. Additionally, they have the potential to spread infectious pathogens to the locations of equipment. This may be more conducive to bacterial colonization, which can develop into serious illnesses if ignored. Efforts aimed at reducing nosocomial infection should be focused in this direction since these factors are modifiable. These are in line with other studies in Turkey [30,31], Kuwait [13], India [32] and Latvia [32].
Interventions such as suprapubic catheters should be used in specific cases, closed drainage systems should be used when feasible, and urinary catheters should only be used when necessary and should be cleaned daily. It's important to remember that hand washing; a straightforward yet effective technique lowers the transmission of nosocomial infections. Gram-negative bacteria accounted for 72.6 percent of all isolated bacteria, whereas Gram-positive bacteria made up just 23.4%.Data presented in this study indicates that the most frequent bacterial isolates from surgical site infections were, Pseudomonas. aeruginosa(22%), Klebsiellaspp. (20%) and Staphylococcus aureus (15%). Similar findings have been seen in Philippines [33] and Turkey [34]. Among urinary tract nosocomial infections E. coli is the most frequent bactreial isolate.
The most common bacteria in bloodstream nosocomial infections were staphylococcus aureus (37.5%) and coagulase-negative staphylococcus (37.5%). Many microorganisms nowadays are resistant to many antimicrobial agents, and under some situations, almost all of them. Healthcare facilities face the challenge of resistance to antimicrobial drugs; yet, hospitals are particularly vulnerable to the spread of microorganisms because of their high susceptibility population [17]. Our study's results on antibiotic sensitivity validated the concerning proportion of bacterial resistance to widely used antibiotics.
Both Gram-positive and Gram-negative bacterial isolates in this investigation showed high levels of resistance to Tetracycline, Trimethoprim-sulphamethoxazole, Ampicillin, Amoxicillin-Clavulanic acid, Chloramphenicol, and Doxycyclin. However, only Gram-positive bacteria exhibited a gentamicin resistance, whereas Gram-negative bacteria only displayed an intermediate level of resistance. Additionally, the findings showed that the resistance rates of Gram-positive and Gram-negative bacteria separated from nosocomial infections to ciprofloxacin, norfloxacine, and ceftriaxone were low (<60%) Low levels of resistance to nalidixic acid and nitrofurantoin were demonstrated by gram-negative bacteria. The range of resistance rates for Gram-positive and Gram-negative bacteria is 50% to 100% and 33% to 100%, respectively. The antibiotics ciprofloxacin, ceftriaxone, nalidixic acid, nitrofurantoin, and norfloxacine were comparatively successful in treating the microorganisms that cause nosocomial infections. This might be the case given that these agents are very new and not widely utilized. However, because of their high cost, medications such as nitrofurantoin, norfloxacine, and nalidixic acid have limited practical usage. They showed little resistance as a result. This study suggests that when it comes to treating nosocomial infections, the practitioner has rather limited options.
Compared to bacterial isolates that were Gram-positive, resistance rates to all antibiotics examined for Gram-negative were generally lower. This is consistent with other research conducted in Thailand (Danchaivijitr, et al. [35]) and Nigeria [36], where the majority of the isolates from non-infectious infections were resistant to widely used antibiotics. The current investigation did, however, reveal a substantial rate of resistance to the antimicrobial drugs that are often administered. This might be the result of the widespread use of antibiotics for self-medication that is accessible over the counter, the widespread availability and careless use of these medications outside of hospitals, and the heavy usage of antimicrobial agents in hospitals.These issues are recognized to be the cause of circulating resistance strains, together with the higher risk of cross-infection among inpatients.
It is important to note that not all Ogun State University Teaching Hospital, Sagamu wards—such as the medical, intensive care, pediatric, and gynecology and obstetrics wards—in which a high incidence of nosocomial infections is suspected—are included in this study. Anaerobic microorganisms could not be included because of financial and facility limitations in the lab. Fungal infections and other pathogens that are significant nosocomial infection causes were not included in the study's design. This research did not cover patients who get nosocomial infections after being released from the hospital.
The recommendations listed below can be implemented in light of these findings: All wards at Ogun State University Teaching Hospital, Sagamu should include anaerobic bacteria, fungi, and other microorganisms to determine the prevalence and medication susceptibility pattern of nosocomial diseases. Drug resistance is a result of empirical therapy for nosocomial infections; as a result, the basis of treatment should be the culture and sensitivity results. To do this, the microbiological lab's capacity has to be increased with qualified personnel, funding, and the required lab supplies. To serve as the foundation for an alternate course of therapy, constant surveillance for resistant bacteria is required. The hospital should be the center of attention for nosocomial infection control, using distinct personnel, resources, and funding. According to this study, Ampicillin, Amoxicillin-Clavulanic acid, Amoxicillin, Methicillin, Tetracycline, Trimethoprim-sulphamethoxazole, Doxycyclin, Chloramphenicol, and Penicillin are somewhat inefficient in treating nosocomial infections if one could not wait for the culture findings.
Compared to the same hospital's prior research, Ogun State University Teaching Hospital, Sagamu had a lower prevalence of nosocomial infections. Gram-negative bacteria accounted for 75% of isolates in SSIs and 95.6 % of UTIs, respectively, whereas Gram-positive bacteria (87.3%) made up the majority of organisms in BSIs. For Gram-positive bacteria, Ceftriaxone and Ciprofloxacin were found to be reasonably effective medications; for Gram-negative bacteria, Norfloxacine, Ceftriaxone, and Nitrofurantoin were reasonably effective medications. However, all of the Gram-positive bacteria isolated from nosocomial infections were resistant to trimethoprim-sulphamethoxazole, ampicillin, tetracycline, and chloramphenicol. Furthermore, all isolates of Gram-negative bacteria were completely resistant to trimethoprim-sulphamethoxazole, tetracycline, and amoxicillin.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the ethical standards obtained from Ogun State University Teaching Hospital, Sagamu and duly signed informed consent was obtained from all participants.
Consent for Publication
Not applicable.
Availability of Data and Material
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Competing Interests
The authors declare that they have no competing interests.
Funding
This review did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' Contributions
Not applicable.
Acknowledgements
I would like to express my sincere gratitude to my Scientific Adviser, Dr Florin D Petrariu from the Department of Hygiene – Environmental Health, Faculty of Medicine, Universitatea de MedicinășiFarmacie "Grigore T. Popa" Iași for providing supervision and constructive advice during the course of this project in 2012. I am grateful to the Staffs and Clinicians of Ogun State University Teaching Hospital Sagamu, for their support in identifying the target patients, sample collections and in facilitating good working environment.
Author’s Information
Dr Adewale Lawrence, Founder/CEO Bioluminux Clinical Research Bioluminux Clinical Research, 720 Brom Drive, Suite # 205, Naperville, Illinois, United States Doctor of Medicine degree with Masters in Regulatory Affairs for Drug, Biologics and Medical Devices. A principal Investigator and therapy area lead for clinical trial studies at Bioluminux Clinical Research Network. Research work was completed in 2012, submitted in partial fulfillment of the requirements for the degree of Doctor of Medicine (MD) at the Faculty of Medicine, Universitatea de MedicinășiFarmacie "Grigore T. Popa" Iași, Romania.


