Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Gian Maria Pacifici*
Received: March 20, 2024; Published: April 04, 2024
*Corresponding author: Gian Maria Pacifici, Professor of Pharmacology, Via Sant’Andrea 32,56127 Pisa, Italy
DOI: 10.26717/BJSTR.2024.55.008762
Clarithromycin is a macrolide antibiotic and has good activity against Moraxella catarrhalis, Chlamydia species, Helicopter pylori, Borrelia burgdorferi, Legionella pneumonia, Mycoplasma leprae, non-tuberculous mycobacteria, and some protozoa such as Toxoplasma gondii, Cryptosporidium, and Plasmodium species. Clarithromycin is absorbed rapidly after oral administration and is metabolized presystemically and 14-hydroxy-(R)-clarithromycin is the main microbiological active metabolite and clarithromycin is also N-demethylated. Clarithromycin has been found efficacy and safe. The prevention and treatment of bacterial infections with clarithromycin and trials conducted with clarithromycin have been reviewed. The pharmacokinetics of clarithromycin have been studied in healthy male volunteers following the single oral dose of 250 mg of clarithromycin and the mean elimination half-life in plasma is 1.9 hours and is 3.7 hours following the oral dose of 500 mg twice-daily. The penetration of clarithromycin into the human tissues has been reviewed and the mean peak concentration of clarithromycin is 1.09, 0.07, and 0.08 μg/ ml in plasma, subcutaneous adipose tissue, and in skeletal muscle, respectively. Clarithromycin and 14-hydoxy-(R)-clarithromycin reach similar concentration in serum and in sputum. Clarithromycin achieves higher concentration in periodontal tissue, epithelial lining fluid and in macrophages than in plasma and Clarithromycin and 14-hydoxy-(R)-clarithromycin achieves higher concentration in alveolar macrophages than in plasma. Clarithromycin interacts with drugs and when clarithromycin is co-administered with colchicine, carbamazepine, or with digoxin causes toxicity. The aim of this study is to review clarithromycin efficacy and safely, prevention and treatment of bacterial infections, trials, metabolism, pharmacokinetics, penetration into tissues, interaction with drugs, and toxicity.
Keywords: Clarithromycin; Drug-Interaction; Efficacy-Safely; Metabolism; Pharmacokinetics; Prophylaxis; Tissue-Concentration; Toxicity; Treatment and Trials
Antimicrobial Activity of Clarithromycin
Clarithromycin is a macrolide antibiotic and has good activity against Moraxella catarrhalis, Chlamydia species, Mycoplasma pneumoniae, Helicopter pylori, Borrelia burgdorferi, Legionella pneumonia, and Mycobacterium leprae. Clarithromycin has enhanced activity against some non-tuberculous mycobacteria, as well as against some protozoa (e.g., Toxoplasma gondii, Cryptosporidium, and Plasmodium species) [1].
Absorption, Distribution, Metabolism and Elimination of Clarithromycin
Clarithromycin is absorbed rapidly from the gastrointestinal-tract after oral administration, but hepatic first-pass metabolism reduces its bioavailability to 50% to 55%. Peak concentration occurs about 2 hours after drug administration. Clarithromycin may be given with or without food, but the extended-release form should be administered with food to improve the bioavailability. Clarithromycin and its active metabolite, 14-hydroxy-(R)-clarithromycin, achieve high intracellular concentrations throughout the body, including the middle ear. Clarithromycin is metabolized in the liver to several metabolites and the active 14-ydroxy metabolite is the most significant. The elimination half-life is 3 to 7 hours for clarithromycin and 5 to 9 hours for 14-hydroxy-clarithromycin. Metabolism is saturable, resulting in nonlinear pharmacokinetics and longer half-lives with higher dosages. The amount of clarithromycin excreted unchanged in the urine ranges from 20% to 40% depending on the dose administered and the formulation (tablet versus oral suspension). An additional 10% to 15% of a dose is excreted in the urine as 14-hydroxy-claritrhinycin. Dose adjustment is not recommended unless the creatinine clearance is less than 30 ml/min [1] (Figure 1).
The literature search was performed electronically using PubMed database as search engine and the following key words were used: “clarithromycin efficacy, safely”, “clarithromycin prophylaxis”, “clarithromycin treatment”, “clarithromycin trials”, “clarithromycin metabolism”, “clarithromycin pharmacokinetics”, clarithromycin tissue concentration”, “clarithromycin drug interactions”, and “clarithromycin toxicity”. In addition, the book: Goodman@Gilman’s. The Pharmacological basis of Therapeutics [1] has been consulted.
Efficacy and Safely of Clarithromycin
Forty-six patients with chest infected by Legionella pneumonia received clarithromycin orally at the dose of 500 to 1,000 mg twice-daily and the response-rates were as follows: clinical cure-rate 98%, clinical success (cure or improved) 100%, radiographic success (cure and improved) 93%, and bacteriological cure 100%. Ten patients reported 13 adverse-effects (7 mild, 4 moderate, and 2 severe). Clarithromycin is an effective and safe treatment of patients with severe chest infections due to Legionella pneumophila [2]. Six patients with pan-bronchiolitis were treated with clarithromycin orally at the daily dose of 200 mg for 4 years and the pulmonary function improved in most of patients within 6 months of treatment. The forced expiratory volume in 1 second showed a maximal increase from 1.74±0.12 at baseline to 2.31±0.22 at 6 months (P-value < 0.01) and the volume of forced vital capacity also showed a maximal increase within 6 months of treatment. The partial pressure of arterial oxygen significantly increased within 3 to 6 months of treatment. The comprehensive improvement scores also reached maximum within 6 months of treatment. Patients maintained a stable condition with continued therapy and no adverse-effects due to clarithromycin were observed. These results indicate that 6-month treatment with clarithromycin is sufficient to improve the clinical conditions of patients with pan-bronchiolitis and the drug was safely used for a long-term treatment [3].
A total of 2,351 patients with acute, mild, or moderate respiratory- tract infection received clarithromycin orally at the dose of 250 mg twice-daily for 7 days. The clinical-rate was 93.2% and 110 adverse-effects (4.7%) were reported. Of these, 71 adverse-effects (64.5%) were related to the gastrointestinal-tract. This treatment was well-tolerated and was an effective therapy for the management of acute, mild, or moderate respiratory-tract infection [4]. Thirty-nine patients with multidrug-resistant tuberculosis, aged 41 years (range, 29 to 49), received clarithromycin orally at the daily dose of 1,000 mg. The minimum inhibitory concentration (MIC) for clarithromycin ranged from < 2 to 8 μg/ml and the serum concentration of clarithromycin was one order of magnitude higher than the MIC for clarithromycin. Clarithromycin was efficacy and well-tolerated in patients with multidrug-resistant tuberculosis [5]. Children, aged ≤ 12 years, with upper respiratory-tract infection received clarithromycin and the treatment was safe and effectively treated children with upper respiratory-tract infection. Clarithromycin was superior to other antibiotics for the treatment of children with upper respiratory-tract infection [6].
Prevention of Bacterial Infections with clarithromycin
Two-hundred-forty pregnant women undergoing non-elective Caesarean delivery were enrolled and 133 women (62.1%) received clarithromycin and 81 women (37.8%) received placebo. Women who received clarithromycin had significantly lower-rate of postpartum endometritis (P-value = 0.025) and significantly lower risk of developing endometritis (P-value = 0.040) than women who received placebo. The surgical prophylaxis with clarithromycin in women undergoing non-elective Caesarean delivery lowers postpartum endometritis and reduces the risk of developing endometritis compared the women who received placebo [7]. It was evaluated the effect of prophylactic clarithromycin in prevention of disseminated Mycobacterium- avium-complex infection in patients with advanced HIV disease. One-hundred-ninety-two HIV-infected patients were enrolled, 84 patients (43.7%) received clarithromycin orally at the dose of 500 mg once-daily, 47 patients (24.5%) received rifabutin orally at the dose of 300 mg once-daily, and 61 patients (31.8%) did not received the prophylaxis. The prophylaxis with clarithromycin was associated with a reduction in the incidence of disseminated Mycobacterium- avium-complex infection, appeared to be superior to rifabutin, and was associated with prolonged survival in patients with advanced HIV disease [8]. It was assessed the effect of prophylactic clarithromycin on Mycobacterium-avium-intracellulare infection in patients with acquired immunodeficiency syndrome. Six-hundred-eight-four patients with Mycobacterium-avium-intracellulare infection were enrolled. The Mycobacterium-avium-intracellulare infection occurred in 31 patients (4.5%) treated with clarithromycin and in 87 patients (12.6%) who received placebo (P-value < 0.001). Clarithromycin prevented the Mycobacterium-avium-intracellulare infection, prolonged survival, and was well-tolerated. [9].
Treatment of Bacterial Infections with Clarithromycin
A 74-year-old woman and a 77-year-old man with rheumatoid arthritis underwent bronchoalveolar lavage which was infected by Mycobacterium-avium. The patients received clarithromycin orally at the daily dose of 1,000 mg for 10 days and both patients were cured [10]. Forty-one patients with erythema migrans received clarithromycin orally at the dose of 500 mg twice-daily for 21 days for treatment of early Lyme disease. Symptoms resolved in 37 patients (90.2%) immediately after the start of treatment and symptoms disappeared in all patients at the end of treatment. Clarithromycin is an effective agent for treatment of early Lyme disease [11]. Clarithromycin was administered orally at the dose of 250 mg twice-daily for 5 to 14 days to 299 patients suffering from dermatologic bacterial infections and clarithromycin effectively treated skin and skin-structure infections [12]. Forty-three patients, aged 32 years (range, 21 to 56), had chronic rhinosinusitis and were treated with clarithromycin orally at the dose of 500 mg twice-daily for 7 days (high-dose) followed by 250 mg twice-daily for 7 days (low-dose). The high-dose of clarithromycin treated chronic rhinosinusitis significantly better (P-value = 0.025) in terms of clinical efficacy than the low-dose. The high-dose of clarithromycin was more effective than the low-dose for treatment of chronic rhinosinusitis [13]. Three-hundred-seventy-six patients with acute exacerbations of chronic bronchitis received either ciprofloxacin or clarithromycin orally at the dose of 500 mg twice-daily for 14 days. The clinical resolution was observed in 89.9% of patients who received ciprofloxacin and in 82.4% of patients who received clarithromycin (P-value > 0.05). The median infection-free interval was 142 days for ciprofloxacin recipients and 51 days for clarithromycin recipients (P-value = 0.015). Bacteriological eradication-rate was 93.6% for ciprofloxacin recipients and 77.0% for clarithromycin recipients (P-value = 0.01). Ciprofloxacin was associated with longer infection-free interval and a higher bacteriological eradication-rate [14].
Trials Conducted with Clarithromycin
A prospective, self-controlled trial was conducted in 18 patients with refractory chronic rhinosinusitis who received clarithromycin orally at the dose of 250 mg once-daily for 12 weeks. Significant improvement was observed in nasal congestion, rhinorrhoea, and postnasal drip (P-value < 0.05) within 4 weeks of treatment. Analysis of middle nasal meatus flora revealed a significant decrease of Streptococcus pneumoniae after 12 weeks of treatment (P-value < 0.05). Low-dose and long-term clarithromycin treatment improved the clinical symptoms in patients with refractory chronic rhinosinusitis and facilitates the nasal mucosal epithelialization [15]. A randomized, placebo-controlled, double-blind trial was conducted with clarithromycin in patients with AIDS. Mycobacterium-avium-complex infection developed in 19 of the 333 patients (5.7%) assigned to clarithromycin and in 53 of the 334 (15.9%) assigned to placebo (P-value < 0.001). During the follow-up period of 10 months, 32.0% of the patients who received clarithromycin died and 41.1% of those who received placebo group died (P-value = 0.026). In patients with AIDS, the prophylactic administration of clarithromycin was well-tolerated, prevented Mycobacterium-avium-complex infection, and reduced the mortality [16]. A randomized, blinded, multicentre trial compared the efficacy and safely of oral clarithromycin and amoxicillin/clavulanate in treatment of acute otitis media in 338 children aged 6 months to 12 years. Children received either clarithromycin 7.5 mg/kg twice-daily (N = 161, 47.6%) for 10 days or amoxicillin/clavulanate 13.3 mg/kg thrice-daily (N = 177, 52.4%) for 10 days. The efficacy was assessed by clinical examination performed within 48 hours of finishing the medication. A successful clinical response was seen in 121 of 135 children (89.6%) who received clarithromycin children and in 133 of 145 children (91.7%) who received amoxicillin/clavulanate (P-value = 0.681). The clinical failure or relapses occurred in 14 of 135 children (10.4%) who received clarithromycin and in 12 of 145 children (8.3%) treated with amoxicillin/clavulanate-treated (P-value > 0.05). Diarrhoea was the most frequent adverse-effect which occurred in 19 of 161 children (11.8%) treated with clarithromycin and in 57 of 177 children (32.2%) who received amoxicillin/clavulanate (P-value < 0.001). These results indicate that the efficacy of clarithromycin was comparable to that of amoxicillin/clavulanate in treatment of children with acute otitis media and clarithromycin induces less diarrhoea than amoxicillin/clavulanate [17]. A multicentre, single-blind, comparative trial was conducted in 379 children, aged 6 months to 12 years, with acute otitis media. Children were randomized to receive either clarithromycin orally at the dose of 7.5 mg/kg once-daily for 10 days (N = 229, 60.4%) or cefaclor orally at the dose of 20 mg/kg twice-daily (N = 150, 39.6%) for 10 days. The clinical success (cure or improvement) was achieved in 86.1% of children who received clarithromycin and in 90.2% of children who received cefaclor (P-value > 0.05) and both drugs were well-tolerated. Clarithromycin and cefaclor were similarly effective for treatment of children with acute otitis media [18]. A prospective, randomized, single-blind, parallel-group trial was conducted to compare the clinical efficacy of clarithromycin versus that of erythromycin in 153 children with pertussis aged 1 month to 16 years. Children received either clarithromycin orally at the dose of 7.5 mg/kg twice-daily for 7 days (N = 76, 49.7%) or erythromycin orally at the dose of 13.3 mg/kg thrice-daily day for 14 days (N = 77, 50.3%). Microbiologic eradication and clinical cure-rates were 100% for clarithromycin and 96.2% for erythromycin (P-value > 0.05). The adverse-effects occurred in 34 of 76 children (44.7%) treated with clarithromycin and in 48 of 77 children (62.3%) treated with erythromycin (P-value = 0.035). A 7-day regimen of clarithromycin and a 14-day course of erythromycin were equally effective for treatment of children with pertussis and clarithromycin was better tolerated than erythromycin [19]. A multicentre, double-blind, randomized trial was conducted to assess the efficacy and safely of clarithromycin versus those of erythromycin stearate in treatment of community-acquired pneumonia. Two-hundred-eight patients were enrolled and 96 patients (46.1%) received clarithromycin orally at the dose of 250 mg twice-daily and 112 patients (53.8%) received erythromycin stearate orally at the dose of 500 mg 4 times-daily. The clinical cure, the clinical success, and the radiological response were similar in patients treated with clarithromycin and in those treated with erythromycin. The clinical cure-rate after two weeks of treatment occurred in 43 patients (47.9%) who received clarithromycin and in 17 patients (15.1%) who received erythromycin stearate (P-value = 0.003), whilst improvement in cough was observed in 93 patients (96.9%) who received clarithromycin and in 89 patients (80.0%) who received erythromycin stearate (P-value = 0.07). The adverse-effects, mainly gastrointestinal, causing the discontinuation of treatment occurred in 4 of 96 patients (4.1%) treated with clarithromycin and in 21 of 12 patients (18.7%) treated with erythromycin (P-value < 0.01). These results indicate that clarithromycin twice-daily was as effective as erythromycin stearate 4 times-daily for treatment of community-acquired- pneumonia and clarithromycin was better tolerated [20].
Metabolism of Clarithromycin
The metabolic fate of clarithromycin was studied following the administration of a single oral dose of 250 mg or 1,200 mg of 14C-clarithromycin to 6 healthy adult males. The peak plasma concentration of clarithromycin averaged to 0.6 μg/ml after the low-dose and to 2.7 μg/ml after the high-dose. The major metabolite found in plasma and in urine was the microbiologically active 14-hydroxy-(R)-clarithromycin. The low-dose and the high-dose of 14C-clarithromycin were administered for 5 days to 6 healthy adult males. Following the low-dose a mean of 38.1% of radioactivity (18.0% as clarithromycin) was recovered in the urine and following the high-dose a mean of 46.2% of radioactivity (29.1% as clarithromycin) was recovered in the urine, with approximately one-third eliminated during the first 24 hours. The urinary and faecal metabolites revealed the involvement of three metabolic pathways, hydroxylation which gives rise to 14-hydroxy-(R)-clarithromycin and 14-hydroxy-(S)-clarithromycin, N-demethylation, and hydrolysis of the cladinose sugar. The overall recovery of metabolites decreased up to 42.0% after the high-dose suggesting that the metabolism of clarithromycin is saturated [21]. The main metabolites of clarithromycin are 14-hydroxy-(R)-clarithromycin a microbiologically active metabolite and 14-hydroxy-(S)-clarithromycin an inactive metabolite and these metabolites are formed by CYP3A4 [22]. Clarithromycin is rapid absorbed following oral administration and the peak concentration is reached within 2 hours after dosing. The bioavailability of clarithromycin is about 50% after the oral administration and clarithromycin is converted into 14-hydroxy- clarithromycin in the liver before entering the systemic circulation. The antimicrobial activity of 14-hydroxy-(R)-clarithromycin is superior to that of the parent compound. Following the oral administration of 250 mg of film-coated clarithromycin, the peak serum concentration of clarithromycin and that of 14-hydroxy-(R)-clarithromycin is 1.5 and 0.8 μg/ml, respectively. There is a progressive increase in serum concentrations of clarithromycin and 14-hydroxy-(R)-clarithromycin with renal impairment so that the clarithromycin dose should be reduced in severe renal impairment (glomerular filtration- rate < 30 ml/min) [23].
Pharmacokinetics of Clarithromycin and Penetration of Clarithromycin into Human Tissues
Traumuller, et al. [24] studied the pharmacokinetics of clarithromycin in plasma, in interstitial-space fluid of subcutaneous adipose tissue (subcutis), and in skeletal muscle of 6 healthy male volunteers, aged from 25 to 37 years, following a single oral dose of 250 mg of clarithromycin. A plastic cannula was inserted into an antecubital vein to monitor the blood concentrations of clarithromycin at defined time points. Concentrations of clarithromycin in subcutis and in skeletal muscle were determined by micro-dialysis. A micro-dialysis probe with a molecular weight cut-off of 20,000 was inserted into one thigh muscle and into the subcutis and the probe was constantly perfused with ringer’s solution at a flow rate of 1.5 μl/min by means of a precision pump. After a 60 min equilibration period, 250 mg of clarithromycin was administered orally to the fasting volunteers. At 12 hours after the initial single dose of 250 mg, each volunteer continued to receive oral clarithromycin at the dosage of 500 mg twice-daily for 3 to 5 days. Table 1 summarizes the pharmacokinetic parameters of clarithromycin following a single oral dose of 250 mg of clarithromycin and Table 2 summarizes the pharmacokinetic parameters of clarithromycin following an oral dose of 500 mg twice-daily.
Table 1: Pharmacokinetic parameters of clarithromycin which have been obtained following the administration of a single oral dose of 250 mg of clarithromycin to 6 healthy male volunteers. Values are the mean±SD by (Traunmüller et al. [24]).
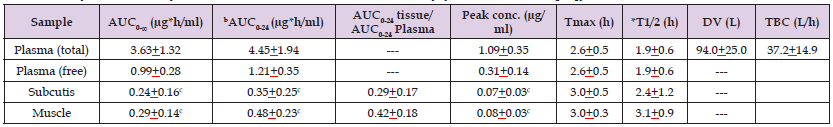
Note: AUC = area under the concentration-time curve. Tmax = time to reach the peak concentration. *Elimination half-life. DV = distribution volume. TBC = total body clearance. bCalculated for single dose of 250 mg. cThe area under the concentration-time curve and the peak concentration of clarithromycin in subcutis and muscle are lower (P-value < 0.03) that those in plasma.
Table 2: Pharmacokinetic parameters of clarithromycin which have been obtained following the oral administration of 500 mg of clarithromycin twice-daily to 6 healthy male volunteers. Values are the mean±SD by (Traunmüller et al. [24]).
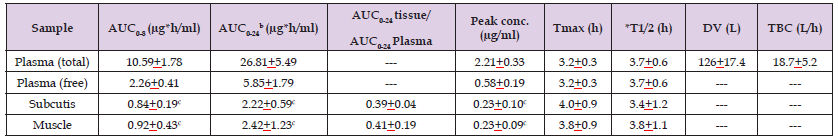
Note: AUC = area under the concentration-time curve. Tmax = time to reach the peak concentration. *Elimination half-life. DV = distribution volume. TBC = total body clearance. bValues calculated on the basis of dosing twice-daily. cThe area under the concentration-time curve and the peak concentration of clarithromycin in subcutis and muscle are lower (P-value < 0.03) those that in plasma.
The area under the concentration-time curve and the peak concentration of clarithromycin are higher in plasma than in subcutis and in muscle. The mean elimination half-life of clarithromycin in plasma is 1.9 hours and is 2.4 and 3.1 hours in subcutis and in muscle, respectively. The concentration of free clarithromycin is lower than the total concentration of clarithromycin. Clarithromycin rapidly penetration into the subcutis and into the muscle as the time to reach the peak concertation is about 3 hours. The distribution volume of clarithromycin is similar to the water volume. This table shows that the area under the concentration-time curve and the peak concentration of clarithromycin in plasma, in subcutis, and in muscle are higher than those obtained following the single oral dose of 250 mg of clarithromycin. The area under the concentration-time curve and the peak concentration of clarithromycin are higher in plasma than in subcutis and in muscle. Clarithromycin rapidly penetrates into the subcutis and muscle as the time to reach the peak concentration is about 4 hours. Clarithromycin is rapidly eliminated as the elimination half-life is about 3.7 hours in plasma, in subcutis, and in muscle and the elimination half-live of clarithromycin in plasma, in subcutis, and in muscle are longer than those obtained following a single oral dose of 250 mg. The metabolism of clarithromycin is saturated thus longer half-life is obtained with higher doses of clarithromycin. The distribution volume of clarithromycin is larger than the water volume and is larger than that obtained following the single oral dose of 250 mg.
Concentrations of Clarithromycin and 14-Hydroxy-( R)-Clarithromycin in Serum and in Sputum
Tsang et al. [25] measured the concentration of clarithromycin and 14-hydroxy-(R)-clarithromycin in serum and in sputum 4, 8, and 24 hours after the administration of a single oral dose of 250 or 500 mg of clarithromycin to 8 patients with idiopathic bronchiectasis having a mean age of 53.3 years. Table 3 summarizes the concentrations of clarithromycin and 14-hydroxy-(R)-clarithromycin in serum and in sputum of 8 patients with idiopathic bronchiectasis. (Raghunatha [26]) administered clarithromycin orally at the dose of 500 mg once daily for 3 days to 20 patients aged 25 to 45 years. Six hours after the last dose, the mean concentrations of clarithromycin in serum and in periodontal tissue were 0.465 μg/ml and 2.61 μg/gram, respectively, indicating that clarithromycin accumulates into the periodontal tissue. (Burrell [27]) administered clarithromycin orally at the dose of 500 mg once-daily for 6 consecutive days to 8 healthy volunteers and experimental gingivitis was induced in these subjects. The mean clarithromycin concentrations in gingiva of healthy control subjects and in inflamed gingiva were 2.4 and 3.0 μg/gram, respectively, and were significantly higher than that found in serum which was 0.5 μg/ ml; P-value < 0.05. Clarithromycin concentrations in gingiva of control subjects and in gingivitis were higher than in serum by 5.7-fold and 7.0-fold, respectively; P-value = 0.02. Clarithromycin attained higher concentration in gingiva than in serum and reached higher concentration in inflamed gingiva than in healthy gingiva.
Table 3: Concentrations of clarithromycin and 14-hydroxy-(R)-clarithromycin which have been obtained in serum and in sputum of 8 patients with idiopathic bronchiectasis. Values are the mean±SD, by Tsang, et al. [25].

Note: CLA = clarithromycin. HCL = 14-hydroxy-(R)-clarithromycin. This table shows that the concentrations of clarithromycin and 14-hydroxy-(R)-clarithromycin are similar in serum and sputum.
Wust and Hardegger [28] administered a single oral dose of 500 mg of clarithromycin to 10 healthy volunteers. Two hours after administration, clarithromycin concentration was 4.04±1.14 μg/ml in serum and 2.72±0.87 μg/ml in saliva. Seven hours after administration, these concentrations were 1.98±0.65 and 1.21±0.34 μg/ml, respectively, and at the end of the dosing interval of 12 hours these concentrations were 0.95±0.38 and 0.73±0.35 μg/ml, respectively. These results indicate that the concentration of clarithromycin is lower (P-value < 0.05) in saliva than in serum. (Gotfried, et al. [29]) administered clarithromycin orally at the dose of 1,000 mg once-daily for 5 consecutive days to 42 non-smoking, healthy volunteers, aged 18 to 54 years. The concentrations of clarithromycin were measured in plasma, epithelial lining fluid, and in the alveolar macrophages and the concentrations of 14-hydroxy-clarithromycin were measured in plasma and in alveolar macrophages. Table 4 shows the steady-state concentrations of clarithromycin in plasma, epithelial lining fluid, and in alveolar macrophages and the concentrations of 14-hydroxy-clarithromycin in plasma and in alveolar macrophages obtained at 6 times after the oral administration of 1,000 mg of clarithromycin to 42 non-smoking healthy volunteers.
Table 4: Steady-state concentrations of clarithromycin which have been obtained in plasma, epithelial lining fluid, and alveolar macrophages and concentrations of 14-hydroxy-clarithromycin which have been obtained in plasma and in alveolar macrophages following the oral administration of 1,000 mg once-daily of clarithromycin to 42 non-smoking, healthy volunteers. Values are the mean±SD, by (Gotfried et al. [29]).
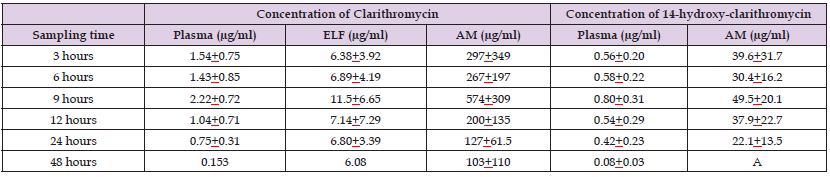
Note: ELF = epithelial lining fluid. AM = alveolar macrophages. ASubjects who had clarithromycin concentration equal or below the quantitative limit of detection. This table shows that the concentrations of clarithromycin in the epithelial lining fluid (P-value < 0.05) and in the alveolar macrophages (P-value < 0.001) are significantly higher than that in plasma and the concentration of clarithromycin in alveolar macrophages is significantly higher (P-value < 0.001) than that in the epithelial lining fluid. The concentration of 14-hydroxy-clarithromycin is significantly higher (P-value < 0.01) in the alveolar macrophages than in plasma.
Interaction of Clarithromycin with Drugs
It was assessed the interaction between clarithromycin and the calcium-channel-blocker amlodipine. Clarithromycin is an inhibitor of CYP3A4 which metabolizes amlodipine thus clarithromycin inhibits the metabolism of amlodipine causing an increase in amlodipine serum concentration [30]. The area under the concentration-time curve of esomeprazole approximately doubled when esomeprazole was co-administered with clarithromycin. Clarithromycin decreased the metabolic-rate of esomeprazole being an inhibitory of CYP2C19 leading to an increase in the serum concentration of esomeprazole [31]. It was assessed the interaction of clarithromycin with rifampicin. Rifampicin induces CYP3A4 which metabolizes clarithromycin thus the co-administration of rifampicin with clarithromycin caused a reduction of the serum concentration of clarithromycin [32]. It was assessed the interaction of clarithromycin with rifampicin. It was measured the plasma concentration of clarithromycin and 14-hydroxy-( R)-clarithromycin in 9 patients before and after the addition of rifampicin. After the addition of rifampicin, the mean plasma concentration of clarithromycin significantly decreased while that of 14-hydroxy-(R)-clarithromycin did not [33]. It was evaluated the pharmacokinetic interaction between clarithromycin and rifabutin. Rifabutin caused a mean reduction of 44% (P-value = 0.003) of the area under the plasma concentration-time curve (AUC) of clarithromycin while caused a mean increase of 57% (P-value = 0.004) in the AUC of 14-hydroxy-clarithromycin.Clarithromycin caused a mean reduction of 99% (P-value = 0.001) of rifabutin AUC and a mean increase of 375% (P-value < 0.001) of the AUC of rifabutin metabolite 25-O-desacetyl-rifabutin. The co-administration of clarithromycin with rifabutin resulted in significant bidirectional pharmacokinetic interactions [34].
Toxicity Induced by Clarithromycin Co-Administered with Drugs
It was assessed the effect of the co-administration of clarithromycin with colchicine in 116 patients. Nine of 88 patients (10.2%) who received clarithromycin and colchicine concomitantly died and only 1 of 28 patients (3.6%) who received the drugs sequentially died. Multivariate analysis of 88 patients who received concomitant therapy showed the presence of renal impairment (relative risk: 9.1; 95% confidence interval: 1.75 to 47.06; P-value < 0.001). Clarithromycin increased the risk of fatal colchicine toxicity especially in patients with renal insufficiency [35]. Carbamazepine is metabolized into carbamazepine- 10,11-epoxide by CYP3A4 and clarithromycin is an inhibitor of CYP3A4 and the serum levels of carbamazepine increased in patients while taking clarithromycin and 3 patients developed toxic serum levels of carbamazepine. There was a serious drug interaction between carbamazepine and clarithromycin thus the dosage of carbamazepine should be reduced by 30% to 50% when carbamazepine is co-administered with clarithromycin and the serum concentration of carbamazepine should be monitored [36]. An epileptic woman 64-year-old was maintained on carbamazepine and received clarithromycin to treat chest infection. Three days after the administration of clarithromycin the serum concentration of carbamazepine became 17.6 μg/ml and this toxic serum concentration of carbamazepine caused symptoms like ataxia, nystagmus, drowsiness, agitation, dilated pupil, respiratory depression, coma and electrolyte disturbances like hyponatraemia and hypokalaemia [37]. A 56-year-old woman with history of severe rheumatic mitral stenosis and atrial fibrillation was treated with digoxin orally at the daily dose of 0.25 mg and she also received clarithromycin orally at the dose of 500 mg twice-daily to treat community-acquired-pneumonia. Two days after clarithromycin administration, she developed profound weakness, associated with nausea, vomiting, dizziness and dyspnea. Clarithromycin inhibits CYP3A4 which metabolizes digoxin thus the serum concentration of digoxin became high and caused toxicity when digoxin is co-administered with clarithromycin [38]. A 78-year-old woman was maintained on digoxin orally at the daily dose of 0.25 mg to treat congestive heart failure and she also received clarithromycin orally at the dose of 500 mg twice-daily. Three days after clarithromycin treatment she developed nausea, vomiting, and diarrhea. The treatment with clarithromycin was discontinued and the toxic symptoms ceased [39].
Clarithromycin is a macrolide antibiotic and has good activity against Moraxella catarrhalis, Chlamydia species, Mycoplasma pneumoniae, Helicopter pylori, Borrelia burgdorferi, Legionella pneumonia, and Mycobacterium leprae. Clarithromycin has enhanced activity against some non-tuberculous mycobacteria, as well as against some protozoa (e.g. Toxoplasma gondii, Cryptosporidium, and Plasmodium species). Clarithromycin is rapidly absorbed from the gastrointestinal- tract but is metabolized presystemically and the bioavailability of clarithromycin is about 50%. The metabolism of clarithromycin is saturable resulting in nonlinear pharmacokinetics and longer half-life occurs following the administration of higher doses of clarithromycin [1]. The efficacy and safely of clarithromycin have been reviewed. Following the oral dose of 500 to 1,000 mg twice-daily of clarithromycin to 46 patients with severe chest infection this drug has been found efficacy and safely and causes adverse-effects in only 10 patients (21.7%) [2]. Patients with pan-bronchiolitis were treated with clarithromycin orally at the daily dose of 200 mg for 4 years and the pulmonary function improved in most patients within 6 months of treatment. Patients maintained stable condition with continued therapy, not adverse-effects were observed, thus 6 month treatment with clarithromycin is sufficient to improve the clinical conditions, and clarithromycin has been found safe for a long-term treatment [3]. Patients with acute, mild, or moderate respiratory-tract infection received clarithromycin orally at the dose of 250 mg twice-daily for 7 days. The clinical-rate was 93.2% and the adverse-effects were reported in only 4.7% of patients. This treatment is well-tolerated and is an effective therapy for the management of respiratory-tract infections [4]. Patients with multidrug-resistant tuberculosis received clarithromycin orally at the daily dose of 1,000 mg and this treatment is found efficacy and well-tolerated [5]. Children with upper respiratory-tract infection received clarithromycin which effectively treats the children and is superior to other antibiotics for the treatment of children with upper respiratory-tract infection [6]. These results indicate that clarithromycin is efficacy and safe in treatment of chest infection caused by Legionella pneumonia, pan-bronchiolitis, and respiratory-tract infection in adults and in children. The prevention of bacterial infections with clarithromycin has been reviewed. Pregnant women undergoing non-elective Caesarean delivery received clarithromycin or placebo. Women who received clarithromycin had lower-rate of postpartum endometritis (P-value = 0.025) and lower risk of developing endometritis (P-value = 0.040) than women who received placebo [7]. Patients with disseminated Mycobacterium-avium-complex and with advanced HIV disease received prophylactic clarithromycin administered orally at the dose of 500 mg once-daily (43.7% patients), or prophylactic rifabutin administered orally at the dose of 300 mg (24.7% patients), or did not received prophylaxis (31.8% patients). Clarithromycin is associated with a reduction in the incidence of disseminated Mycobacterium-avium-complex, is superior to rifabutin, and prolongs survival in these patients [8]. Patients with Mycobacterium-avium-intracellular infection and with acquired-immunodeficiency-syndrome received prophylactic clarithromycin or received placebo. The Mycobacterium- avium-intracellulare infection occurs in 4.5% of patients treated with clarithromycin and in 12.6% of patients who received placebo (P-value < 0.001). Clarithromycin prevents the Mycobacterium- avium-intracellular infection, prolongs survival in these patients, and is well-tolerated [9]. These results indicate that the prophylaxis with clarithromycin reduces the postpartum endometritis and lowers the risk of developing endometritis in women undergoing non-elective Caesarean delivery, prevents disseminated Mycobacterium-avium- intracellular infection in patients with HIV disease, and prolongs survival in patients with Mycobacterium-avium-intracellular infection. The treatment of bacterial infections with clarithromycin has been reviewed. An old woman and an old man had the bronchoalveolar lavage infected by Mycobacterium-avium, received clarithromycin orally at the daily dose of 1,000 mg for 10 days and the bronchoalveolar lavage became sterile [10]. Patients with erythema migrans received clarithromycin orally at the dose of 500 mg twice-daily for 21 days to treat early Lyme disease. This treatment cured 90.2% of patients immediately after the start of treatment and symptoms disappeared in all patients at the end of treatment [11]. Clarithromycin was administered orally at the dose of 250 mg twice-daily for 5 to 14 days to patients suffering from dermatological infections and this treatment effectively treats skin and skin-structure infections [12]. Patients with chronic rhinosinusitis received clarithromycin orally at the dose of 500 mg twice-daily for 7 days (high-dose) followed by 250 mg twice-daily for 7 days (low-dose). The high-dose is more effective (P-value = 0.025) than the low-dose for treatment of chronic rhinosinusitis [13]. Patients with acute exacerbations of chronic bronchitis received either ciprofloxacin or clarithromycin orally at the dose of 500 mg twice-daily for 14 days. The clinical resolution of symptoms was similar in patients who received ciprofloxacin and in those who received clarithromycin but the median infection-free interval was 142 days for ciprofloxacin recipients and 51 days for clarithromycin recipients (P-value = 0.015) and the eradication-rate was 93.6% for ciprofloxacin recipients and 77.0% for clarithromycin recipients (P-value = 0.01). Ciprofloxacin is associated with longer infection-free interval and with higher bacteriological eradication-rate [14]. These results indicate that clarithromycin sterilizes the bronchoalveolar lavage infected by Mycobacterium-avium, treats patients with Lyme disease, patients with skin and skin-structure infections, with chronic rhinosinusitis and treats the chronic bronchitis as ciprofloxacin. The trials conducted with clarithromycin have been reviewed. A prospective, self-controlled trial was conducted in patients with chronic rhinosinusitis who received clarithromycin orally at the dose of 250 mg once-daily for 12 weeks. Nasal congestion, rhinorrhoea, and postnatal drip improved (P-value < 0.05) within 4 weeks of treatment and the decrease of Streptococcus pneumoniae density in middle nasal meatus was observed after 12 weeks of treatment (P-value < 0.05). Lowdose and long-term clarithromycin treatment improves refractory chronic rhinosinusitis and facilities the nasal epithelization [15]. A randomized, placebo-controlled, double-blind trial was conducted with clarithromycin in patients with AIDS. Mycobacterium-avium- complex infection developed in 5.7% of patients who received clarithromycin and in 15.9% of patients who received placebo (P-value < 0.001). During 10 months of follow-up, 32.0% of patients who received clarithromycin and 41.1% of patients who received placebo (P-value = 0.026) died. Clarithromycin is well-tolerated, prevents Mycobacterium- avium-complex, and reduces the mortality [16]. A randomized, blinded, multicentre trial compared the safely and efficacy of clarithromycin administered orally at the dose of 7.5 mg/kg for 10 days versus those of amoxicillin/clavulanate administered orally at the dose of 13.3 mg/kg thrice-daily for 10 days in children with acute otitis media. The successful clinical response and the clinical failure or relapses were similar in both treatments. Diarrhoea was the most frequent adverse-effect and occurred in 11.8% of children who received clarithromycin and in 32.2% of children who received amoxicillin/ clavulanate (P-value < 0.001). Clarithromycin is effective as amoxicillin/clavulanate in treatment of acute otitis media and induces less diarrhoea [17]. A multicentre, single-blind, comparative trial was conducted in children with acute otitis media who received either clarithromycin orally at the dose of 7.5 mg/kg once-daily for 10 days or ceflacor orally at the dose of 20 mg/kg twice-daily for 10 days. Clarithromycin and ceflacor are similar effective in treatment of acute otitis media and both drugs are well-tolerated [18]. A prospective, randomized, single-blind, parallel-group trial was conducted in children with pertussis who received clarithromycin orally at the dose of 7.5 mg/kg for 7 days or erythromycin orally at the dose of 13.3 mg/kg thrice-daily for 14 days. The clinical cure was excellent in both treatments and the adverse-effects occurred in 44.7% and in 62.3% of children (P-value = 0.035) who received clarithromycin and erythromycin, respectively. A 7-day regimen of clarithromycin and a 14-day course of erythromycin are equally effective for treatment of children with pertussis and clarithromycin is better tolerated than erythromycin [19]. A multicentre, double-blind, randomized trial was conducted in patients with community-acquired-pneumonia who received either clarithromycin orally at the dose of 250 mg twice-daily or erythromycin stearate orally at the dose of 500 mg 4 times-daily. The clinical cure, the clinical success, and the radiological response were similar in patients treated with clarithromycin and in those treated with erythromycin but the clinical cure-rate after two weeks of treatment occurred in 47.9% and in 15.1% of patients who received clarithromycin and erythromycin stearate (P-value = 0.003), respectively, whilst improvement in cough was similar in both treatments. Adverse- effects, mainly gastrointestinal, occurred less frequently in patients treated with clarithromycin than in those who received erythromycin stearate (P-value < 0.01). Twice-daily clarithromycin is affective as 4 times-daily erythromycin stearate in treatment of community-acquired-pneumonia and clarithromycin is better tolerated [20]. The metabolism of clarithromycin has been reviewed. Following the administration of a single oral dose of 250 mg or 1,200 mg of 14C-clarithromycin to healthy men volunteers the plasma concentration of clarithromycin averaged to 0.6 and 2.7 μg/ml after the lowdose and the high-dose, respectively. The major metabolite found in plasma and in urine is the microbiologically active 14-hydroxy-( R)-clarithromycin and clarithromycin is also N-demethylated. The overall recovery of clarithromycin decreased up to 42.0% after the high-dose suggesting that the metabolism of clarithromycin is saturated [21]. The mean metabolites of clarithromycin are 14-hydroxy-( R)-clarithromycin a microbiologically active metabolite and 14-hydroxy-(S)-clarithromycin an inactive metabolite and both metabolites are formed by CYP3A4 [22]. Clarithromycin is rapidly absorbed following oral administration and the peak concentration is reached within 2 hours after dosing. Clarithromycin is metabolized presystemically in the liver and the bioavailability of clarithromycin is about 50% after oral administration. Following the oral administration of 250 mg of film-coated clarithromycin, the peak concentration of clarithromycin and that of 14-hydroxy-(R)-clarithromycin is 1.5 and 0.8 μg/ml, respectively. There is a progressive increase in serum concentrations of clarithromycin and 14-hydroxy-(R)-clarithromycin with renal impairment so that the clarithromycin dose should be reduced in severe renal impairment [23]. Traunmuller, et al. [24] studied the pharmacokinetics of clarithromycin in plasma and the penetration of clarithromycin into human interstitial-space fluid of subcutaneous adipose tissue (subcutis), and into the skeletal muscle. Following the administration of a single oral dose of 250 mg of clarithromycin to 6 healthy male volunteers, the elimination half-life of clarithromycin in plasma is 1.9±0.6 hours and following the oral administration of 500 mg of clarithromycin twice-daily the elimination half-life of clarithromycin in plasma is 3.7±0.6 hours. The metabolism of clarithromycin is saturable, resulting in longer half-life with higher doses. Following a single oral dose of 250 mg of clarithromycin to 6 healthy male volunteers, the peak concentration of clarithromycin is 1.09±0.35, 0.07±0.03, and 0.08±0.03 μg/ml in plasma, in subcutis, and in skeletal muscle, respectively, thus the peak concentration of clarithromycin is higher in plasma (P-value < 0.03) than in subcutis and in skeletal muscle. These results indicate that clarithromycin poorly penetrated into the subcutis and skeletal muscle. The elimination half-life of clarithromycin is 1.9±0.6, 2.4±1.2, and 3.1±0.9 hours in plasma, in subcutis, and in skeletal muscle, respectively, indicating that clarithromycin is eliminated faster in plasma than in subcutis and in skeletal muscle. Following the oral administration of 500 mg twice-daily to 6 healthy male volunteers the peak concentration of clarithromycin is 2.21±0.33, 0.23±0.10, and 0.23±0.09 μg/ml in plasma, in subcutis and in skeletal muscle, respectively, indicating that clarithromycin poorly penetrates into the subcutis and skeletal muscle. The elimination half-life of clarithromycin is 3.7±0.6, 3.4±1.2, and 3.8±1.1 hours in plasma and in subcutis and in skeletal muscle, respectively. These half-lives are longer than those obtained following a single oral dose of 250 mg of clarithromycin. The metabolism of clarithromycin is saturable, resulting in longer half-live with higher doses. (Tsang et al. [25]) measured the concentration of clarithromycin and 14-hydroxy-(R)-clarithromycin in serum and in sputum of 8 patients with idiopathic bronchiectasis following a single oral dose of 250 or 500 mg of clarithromycin and found that the concentrations of clarithromycin and 14-hydroxy-(R)-clarithromycin are similar in serum and in sputum. Raghunatha and George [26] administered clarithromycin orally at the dose of 500 mg once-daily to 20 patients and found that the mean concentration of clarithromycin is 0.465 and 2.61 μg/ml in serum and periodontal tissue, respectively, indicating that clarithromycin accumulates in the periodontal tissue. Burrell and Walters [27] administered clarithromycin orally at the dose of 500 mg once-daily for 6 days to 8 healthy volunteers and experimental gingivitis was induced in these subjects. The mean clarithromycin concentrations in healthy control subjects and in inflamed gingiva were 2.4 and 3.0 μg/gram, respectively, and were higher (P-value < 0.05) than that in serum which was 0.5 μg/ml. These results indicate that clarithromycin accumulates in gingiva. Wüst and Hardegger [28] administered a single oral dose of 500 mg of clarithromycin to 10 healthy volunteers. Two hours after dosing, the concentration of clarithromycin was 4.04±1.14 and 2.72±0.87 μg/ml in serum and in saliva, respectively, after 7 hours, these concentrations were 1.98±0.65 and 1.21±0.34 μg/ml, respectively, and after 12 hours these concentrations were 0.95±0.38 and 0.73±0.35 μg/ml, respectively. These results indicate that the concentration of clarithromycin is lower in saliva than in serum. Gotfried et al. [29] administered clarithromycin orally at the dose of 1,000 mg once-daily for 5 consecutive days to 42 non-smoking healthy volunteers and measured the concentration of clarithromycin in plasma, in epithelial lining fluid, and in the alveolar macrophages for 6 times, ranging from 3 to 48 hours, after the dose. The concentration of clarithromycin is higher in the epithelial lining fluid (P-value < 0.05) and in the alveolar macrophages (P-value < 0.001) than in plasma. These authors also measured the concentration of 14-hydroxy-(R)-clarithromycin in plasma and in alveolar macrophages and the concentration of 14-hydroxy-(R)-clarithromycin was higher (P-value < 0.01) in alveolar macrophages than in plasma. These results indicate that clarithromycin accumulates in the epithelial lining fluid and in the alveolar macrophages and 14-hydroxy-( R)-clarithromycin accumulates in the alveolar macrophages. The interaction of clarithromycin with drugs has been reviewed. Clarithromycin is an inhibitor of CYP3A4 which metabolizes amlodipine thus clarithromycin inhibits the metabolism of amlodipine causing an increase in amlodipine serum concentration [30], clarithromycin is an inhibitory of CYP2C9 which metabolizes esomeprazole thus clarithromycin decreases the metabolic-rate of esomeprazole increasing the serum concentration of esomeprazole [31], rifampicin induces CYP3A4 which metabolizes clarithromycin thus rifampicin reduces the plasma concentration of clarithromycin [32], rifampicin reduces the plasma concentration of clarithromycin while does not alter that of 14-hydroxy-(R)-clarithromycin [33], rifabutin reduces the area under the concentration-time curve (AUC) of clarithromycin, increases the AUC of 14-hydroxy-clarithromycin and clarithromycin reduces the AUC of rifabutin and increases the AUC of 25-O-desacetyl-rifabutin, a metabolite of rifabutin [34].
The toxicity induced by clarithromycin co-administered with drugs has been reviewed. The co-administration of clarithromycin with colchicine causes death in 10.2% of patients who received both drugs concomitantly and causes death in 3.6% of patients who received both drugs sequentially [35], carbamazepine is metabolized into carbamazepine-10,11-epoxide by CYP3A4 and clarithromycin is an inhibitor of CYP3A4 and the co-administration of clarithromycin with carbamazepine causes high concentration of carbamazepine which induces toxicity [36], an epileptic woman was maintained on carbamazepine and received clarithromycin to treat chest infection and the serum concentration of carbamazepine became 17.6 μg/ml and this toxic concentration of carbamazepine induced symptoms like ataxia, nystagmus, drowsiness, agitation, dilated pupil, respiratory depression, coma, and electrolytic disturbances like hyponatraemia and hypokalaemia [37], a woman received digoxin orally at the daily dose of 0.25 mg and she also received clarithromycin orally at the dose of 500 mg twice-daily and two days after clarithromycin administration she developed profound weakness associated with nausea, vomiting, dizziness and dyspnoea. Clarithromycin inhibits CYP3A4 which metabolizes digoxin thus the serum concentration of digoxin became high and caused toxicity [38], a woman was maintained on digoxin orally at the daily dose of 0.25 mg and she also received clarithromycin orally at the dose of 500 mg twice-daily and three days after clarithromycin administration she developed nausea, vomiting, and diarrhoea [39]. In conclusion, Clarithromycin is a macrolide antibiotic and has good activity against Moraxella catarrhalis, Chlamydia species, Helicopter pylori, Borrelia burgdorferi, Legionella pneumonia, Mycoplasma leprae, non-tuberculous mycobacteria, and some protozoa such as Toxoplasma gondii, Cryptosporidium, and Plasmodium species. The efficacy and safely of clarithromycin, the prevention and treatment of bacterial infections with clarithromycin have been reviewed. The trials conducted with clarithromycin and the metabolism of clarithromycin have been reviewed. Clarithromycin in metabolized into the microbiologically active 14-hydroxy-(R)-clarithromycin and into the inactive 14-hydroxy-(S)-clarithromycin and clarithromycin is also N-demethylated. The pharmacokinetics of clarithromycin have been reviewed. After a single oral dose of 250 mg of clarithromycin, the elimination half-life of clarithromycin in plasma is 1.09±0.35 hours and following the oral administration of clarithromycin at the dose of 500 mg twice-daily the elimination halflie of clarithromycin in plasma is 3.7±0.6 hours. The metabolism of clarithromycin is saturable thus longer half-life is observed following the administration of high doses. The penetration of clarithromycin and 14-hydroxy-(R)-clarithromycin into human tissues has been reviewed. Clarithromycin interacts with drugs and when clarithromycin is co-administered with colchicine, carbamazepine, or with digoxin causes toxicity. The aim of this study is to review the clinical pharmacology of clarithromycin.
The authors declare no conflicts of financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employments, gifts, and honoraria. This article is a review and drugs have not been administered to men or animals.
The author thanks Dr. Patrizia Ciucci and Dr. Francesco Varricchio, of the Medical Library of the University of Pisa, for retrieving the scientific literature.


