Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Romulo Mendoza*, Nailya Delellis, Ugo Ezenkwele and Horatio Yeung
Received: March 22, 2024; Published: March 27, 2024
*Corresponding author: Romulo Mendoza, School of Health Sciences, Central Michigan University, Mount Pleasant, Michigan, USA
DOI: 10.26717/BJSTR.2024.55.008759
Objective: To identify the most common causes of delays between specimen collection in the ED and
submission of specimens to the laboratory by using available technological devices in the pre-analytical phase
and evaluate the impact of work schedule or shift on the delays.
Methods: In order to identify the common causes of specimen delays, a field experiment was conducted at
the laboratory and the ED of the Mount Sinai Hospital at Queens, New York, from August to November 2018
(4 months) using real-time data posted in the hospital information system by the Epic Rover once ED HCWs
collected the specimens.
Results: The four-month field experiment generated a total of 133 laboratory interventions (calls made by
CPA clerks to the collector of specimens that were not submitted to the lab after 21 minutes of collection to
inquire about the submission delay) that involved 60 nurses. The study categorized the outcome of the lab
interventions into two:
a) Delayed specimen received after the intervention (N = 82, 61.7%), and
b) No specimens received prompting the lab to cancel (N = 51, 38.3%).
We identified the top two reasons for delayed specimen delivery to the lab as
1) Distraction of the health care worker (HCW) who collected the specimens and
2) Unavoidable patient conditions such as difficult veins or unable to void urine.
The top two cancellation reasons were
1) ED request and
2) Patient discharged.
There was no statistically significant association between shift and outcome of the lab interventions (χ₂ (N =
133, 1) = 0.104, p = 0.747).
Conclusions: ED HCW from the day and night shifts faced the same challenges in the pre-analytical phase,
specifically, distraction and unavoidable patient conditions that delay the turnaround time (TAT).
Keywords: Analyte; Turn-Around-Time; Laboratory Testing Workflow; Preanalytical Phase; Epic Rover; Vocera Phones; Order Status Monitor
Abbreviations: TAT: Turnaround Time; HIS: Hospital Information System; MSQ: Mount Sinai Hospital at Queens; LIS: Laboratory Information System; SCC: Specialist Computer Centres; HIS: Hospital Information System; PTS: Pneumatic Tube System; IT: Information Technology; IOS: iPhone Operating System; SCC: Specialist Computer Centres; OSM: Order Status Monitor; CLT: Clinical Laboratory Technologist; PTS: Pneumatic Tube System; LDH: Lactate Dehydrogenase; PIP: Process Improvement Project; OSM: Order Status Monitor; SEIPS: Systems Engineering Initiative for Patient Safety
Laboratory information aids healthcare professionals make proper diagnostic or therapeutic decisions, assisting with a prompt disposition for their patients (Eaton, et al. [1]). Accurate and timely reporting of laboratory analytes to ordering providers plays a significant role in hospital care quality (Cavallo, et al. [2,3]). Critical areas such as the ED rely on laboratory analytes’ expedited results to confirm an initial diagnosis that leads to a shorter length stay. It is essential to monitor the entire laboratory testing workflow’s efficiency because 60% to 70% of the objective information on the patient’s chart is laboratory information (Holland, et al. [4]). However, hospitals currently spend less than 5% of the hospital budget on clinical laboratory services (Eaton, et al. [1]). The hospital laboratory testing workflow is a multi-disciplinary process that begins with computer entry, specimen collection, and specimen transportation (the pre-analytical phase) by various HCW immediately followed by the laboratory procedures to detect and measure biologic substances (the analytical phase) and ends with the electronic transmission of the final laboratory results to the hospital information system (HIS) (the post-analytical phase) (Dasgupta, et al. [5]).
Historically, errors were more predominant in the analytical phase due to all the manual laboratory procedures such as pipetting, titrating, colorimetric readings, and reagent preparation that require frequent troubleshooting. Nowadays, technological advances in laboratory instrumentation, improved reagent stability, and computerized reporting of laboratory results have steadily streamlined the specimen workflow and significantly decreased errors in both analytical and post-analytical phases (Bickley, et al. [6,7]). However, the pre-analytical phase’s error rate remains relatively high (Hammerling, et al. [8-10]). Hammerling [8] studied the laboratory quality assessment and reported that the pre-analytical phase of the total specimen workflow is where the majority of the errors occurred. Plebani [10] also reported that almost 70% of all laboratory diagnostics mistakes are pre-analytical errors that arise from problems in patient preparation, sample collection, transportation, specimen preparation, and specimen storage. According to Hammerling [8], types of errors at the pre-analytical phase could include: inappropriate test request, order entry errors, misidentification of patient, improper container, sample collection and transport inadequate, inadequate sample/anticoagulant volume ratio, insufficient sample volume, sorting and routing errors, and labeling errors.
In general, there are four categories of pre-analytical variability,
including:
1) Test ordering,
2) Patient preparation,
3) Specimen collection, and
4) Specimen processing, transportation, and storage (Ghaedi,
et al. [11,12]).
Unlike the other two phases in the specimen workflow, due to the nature of tasks involved in the pre-analytical phase, such as computer entry of appropriate lab tests, blood collection, patient preparation in some cases, and specimen transportation, it is hard to utilize automation technology to decrease the number of errors (Hammerling, et al. [8-10,13]). Furthermore, it should be noted that common sources of pre-analytical errors occur in settings outside the laboratory’s jurisdiction. Often, the chaotic conditions in the ED sidetrack personnel from routine tasks, which impede the timely submission of collected samples to the laboratory and increase the possibility of making errors (Hawkins [14]). A study on ED physicians and nurses reported a mean rate of 7.51 interruptions per hour with ED colleagues’ interruptions, and telephone/beepers accounted for almost 75% of all interruptions (Weigl, et al. [13]). Proper specimen collection using positive patient identification immediately followed by timely submission to the laboratory for testing are major pre-analytical concerns outside the laboratory’s jurisdiction (Plebani, et al. 2014). Delays on specimen collection and transport could potentially prolong a provider’s time to make a disposition, especially in ED, where multi-tasking nurses are distracted continuously (Lou, et al. [15]).
Currently, the literature search did not reveal any solutions to effectively decrease the errors at the pre-analytical phase and identify and intervene on delayed specimen collection and submission to the laboratory (outliers) in real-time. Thus, this field experiment was designed to identify the common sources of delays in specimen delivery from ED to the lab in the pre-analytical phase. In addition, given that interruption is often listed as a cause for fallouts on other tasks such as specimen collection and specimen transport and assuming a higher level of activities during the day shift compared with the night shift, the study also evaluated the impact of work schedule or shift on the delays by comparing the frequency of negative outcomes between day and night shift. Finding the major sources of delays from the specimen collection at the ED to delivery to the laboratory is the main purpose of this field experiment so that inter-disciplinary leadership teams can plan an applicable system change. Collaborative input from healthcare professionals and laboratory professionals helps identify the major challenges and, at the same time, discuss a resolution to prevent a recurrence.
Research Design
A fast-paced ED environment surrounded by interruptions can generate unintended outliers in the specimen collection process, causing delays in the total turn-around time. In order to identify the common causes of specimen delays, a field experiment was conducted at the laboratory and the ED of the Mount Sinai Hospital at Queens (MSQ), New York, from August to November 2018 (4 months) using real-time data posted in HIS by the Epic Rover once ED HCWs collected the specimens. MSQ is a community hospital in a middle-class, commercial neighborhood of western Queens, NY. MSQ joins six other hospitals and one renowned medical school forming the Mount Sinai Health System, one of the country’s largest nonprofit systems. MSQ recently increased the ED throughput at the new state-of-the-art Emergency Department that opened its doors in May 2016 and cared for 60,000 visits in that year compared to 51,737 in the previous year (Epic). ED visits at MSQ still steadily increases through the years with 63,563, 67,406, and 68,553 from 2017, 2018, and 2019, respectively.
Instruments
This field experiment utilized a combination of existing technological devices, including the Epic Rover, the computer systems starting with the laboratory information system (LIS) which is the Specialist Computer Centres (SCC), interfacing with the hospital information system (HIS) which is Epic, the pneumatic tube system (PTS) manufactured by Swisslog, and the Vocera phones, to study the effects of laboratory interventions.
The Epic Rover
Epic Systems loaded a mobile device (Epic Rover) with accessories such as scanners for barcode validations and software applications that allow a variety of healthcare workers to access and update patient charts directly to HIS (Gramling [16]). The Epic Rover shows applicable medication advisories at the point of care, supports recording vitals, and provides a clinical summary of allergies, labs, and current medications, allowing clinicians to update administrative details such as dose, route, or site. ED nurses at MSQ used the Epic Rover to electronically document every specimen collection that transmitted all the information to the HIS in real-time. Epic Rover relays all the updates in HIS, in which the hospital Information Technology (IT) department interfaced with different computer systems such as the LIS. Specifically, the Epic Rover device requires scanning wristbands from patients for positive identification that eliminated mislabeling, which is a fatal type of pre-analytical error. Mislabeled specimens led to medical errors when laboratory data from one patient was used by a provider to treat another patient. Epic Rover can help HCWs positively identify patients for blood collection and other tasks in patient care such as documentation of vital signs, the performance of pointof- care testing, and administration of medication.
The laboratory can monitor the specimen collection information sent by Epic Rover to HIS and LIS during the pre-analytical phase. It is available for iPhone Operating System (IOS), Android, and other mobile platforms that connect to HIS, allowing access to clinical data but, most importantly, for the positive patient and specimen identification (Gramling [16]). The specimen collectors use Epic Rover to scan the patient’s wristband, displaying all of the patient’s uncollected laboratory orders requested by the provider. The specimen barcode label, which contains patient demographics and a list of tests, prints after selecting all tests to be collected, which the specimen collectors used to confirm patient identification before initiating the specimen collection. Completing the required steps in the specimen collection using Epic Rover sends accurate information to HIS including the user code of the person who collected the specimen, the site of collection (which arm), method of collection (arterial, venipuncture, or fingerstick), and the date and time of collection. Occasionally, HCW’s revert to paper requisitions instead of the Epic Rover during computer downtime. The manual process of paper requisitions is prone to mistakes, primarily due to illegible HCW’s handwriting.
Incorrect entry of the collection time, mostly when HCWs failed to use military time, and laboratory clerks were unable to recognize the error, erroneously displays the results in the wrong sequence on the patient’s flowchart in HIS that could contribute to medical error. Epic Rover automatically transmits the specimen collector’s user code and current date and time to HIS and LIS eliminating any risk of incorrect flowchart sequence.
The Laboratory Information System and Hospital Information System
The Specialist Computer Centres (SCC) is the LIS that enabled a flatscreen to display all collected samples waiting for arrival in the CPA. CPA clerks monitored the flatscreen that refreshed every three minutes. There are several advantages of utilizing the LIS. First, it helped ED nurses to promptly collect specimens with required collection kits and proper collection techniques, including the full utilization of the Epic Rover. Through LIS, the CPA clerks were able to capture the real-time collection information from the Epic Rover through the wall-mounted flatscreen and connect to more nurses as they frequently checked the order status monitor (OSM) displayed on a wall-mounted flatscreen inside the CPA workstation for a specimen collected but not received (outliers). The LIS also streamlined the information delivery to HIS. After CPA clerks processed all specimens received, which they delivered to the workstations of the clinical laboratory technologist (CLT) team for analysis using either manual or automated methodologies, CLTs reviewed all laboratory instrument output and finalized test result interpretations, which were then sent over by interface from the LIS to HIS. Epic is the HIS used mainly by the rest of the hospital HCWs. Epic stores all patient information needed by providers and other HCWs to deliver safe and quality patient care.
The Pneumatic Tube System
MSQ used the pneumatic tube system (PTS) manufactured by Swisslog Healthcare, Westerstede, Germany, an efficient and cost-effective mode of specimen transport from the ED to the laboratory. PTS canisters contain inner foam inserts and travel at a constant speed of approximately 20 ft/sec with a distance of about 200 feet between the lab and the ED stations. According to Swisslog, a study by the Ohio State Medical University concluded that the PTS could save 160 work hours each day. Using the PTS allowed ED nurses and other HCWs to perform additional clinical functions instead of spending time transporting specimens to the laboratory. However, several features of PTS, such as speed, distance, and packing material that might contribute to hemolysis, so Phelan, et al. (2017) conducted a study to prove the acceptability of using PTS regarding hemolysis. The research of Phelan, et al. (2017) showed a minimal variation of the hemolysis rate between the use of PTS (12.6%) and hand-delivery (14.6%). A similar study conducted by Cui, et al. [17] concluded that using PTS canisters without the foam inserts had a statistical effect on both the lactate dehydrogenase (LDH) and the potassium levels. Another study conducted by Cakirca, et al. [18] also concluded that the samples transported using PTS without the foam inserts showed a significantly higher hemolysis rate (47%) compared to hand-delivered specimens (10%) and PTS using canisters with foam inserts (8%). Users must properly use PTS to reap the benefits of a faster and cost-effective mode of specimen delivery without compromising specimen integrity through hemolysis. Institutions must also have an effective contingency plan when PTS malfunctions because any instrumentation is prone to meet intermittent operational disruptions, a dilemma that happens several times a year. Some PTS malfunctions were resolved remotely by the manufacturer, but there were 29 instances since the July 2016 installation that MSQ hospital reached out to Swisslog to restore service.
The Vocera Phones
Vocera phones are handsfree wearable communication devices that enhanced the hospital system’s mode of interaction (Mack [19]). Vocera phones offer hospitals and health systems communication services by creating hospital-specific smartphones featuring medical alarms and text messaging capabilities (Mack [19]). Vocera phones were assigned to the ED nurses and served as the direct line of communication between ED nurses and CPA clerks. These phones eliminate the CPA clerks’ wasted time calling the nursing station and asking clerical staff to search for the nurse and put him/her on the line. The laboratory’s accessibility to Vocera phones quickly links the nurse with the laboratory technologists during the notification of suboptimal specimens in the pre-analytical phase.
Procedures
We conducted a field experiment that turned out to be a joint process improvement project (PIP) between ED and laboratory for four months on the challenges of timely specimen collection and transport from the ED nursing station to the CPA of the laboratory. The field experiment involved multiple HCWs with specific roles using respective tools and technology to work in a system centered on patient care, as shown in Table 1. During the study period, leaders of ED and the laboratory collaborated on the field experiment to maximize the use of the Epic Rover in the specimen collection process and using paper requisitions only during systemwide computer downtime. The hospital information technology department assisted in installing a large wall-mounted flatscreen that computer analysts interfaced with software to monitor specimen activity. The ED treatment team, led by providers, initiated the specimen workflow through appropriate laboratory orders entered in the HIS. ED nurses use an electronic device similar to a smartphone (Epic Rover) retrofitted by the Epic company with a scanner and software for identification of patients and document various tasks such as vital signs and specimen collection. The entire hospital facility’s specimen collection activities were filtered to capture high priority laboratory tests sent over by the Epic Rover collection devices that interfaced with HIS and LIS.
The implementation of pre-analytical devices such as the Epic Rover enabled laboratories to see incoming specimens and monitor specimen collection and transport progress. Epic Rover registered the date, time, and user code of the person who collected the specimens then transmits all the information to both the HIS and the LIS in real-time. The Epic Rover provided the solution for patient identification problems using scanning technology from a smartphone-like device interfaced with HIS that prevents processing information on misidentified patients. Epic Rover also allowed HCWs to safely simplify and manage their daily tasks using positive patient identification while securely protecting PHI (Talukder, et al. [20]). Meanwhile, laboratory clerks monitored the order status monitor (OSM) linked to HIS that displays all specimens collected by ED HCWs with three minutes of update time. The color-coded notification system under the TAT column (TAT is the abbreviation of “turnaround time”) changes the status to yellow when the laboratory has not received that specimen on the 11th minute and turns red on the 21st minute, as shown in Figure 1. Any specimens collected but not received after 21 minutes were considered outliers and turned red on the OSM, which prompted the CPA clerks to initiate the intervention by calling the HCW who collected the specimens using Vocera phones to inquire about the submission delay.
Vocera phones assigned to every nurse served as a direct link between the laboratory clerks and the ED HCW and enhanced the communication between CPA clerks and ED nurses by eliminating the traditional calls to the nursing station, usually staffed by ED clerks who had to search for the ED nurses. Each phone call represented the laboratory intervention that CPA clerks documented in a PIP log, as illustrated in Figure 2. The PIP log allowed the laboratory to document all communications with the ED personnel for future analysis. The field experiment utilized information from the Epic Rover to open the line of communication via Vocera phones between laboratory clerks and ED nurses regarding specimens that were collected but not received, which then allowed the CPA clerks to intervene by calling the specimen collector if the collected specimens never reached the laboratory within 21 minutes. Non-laboratory personnel performed several activities in the pre-analytical phase that affected the total TAT of the specimen workflow. Specimen collection and transport to the laboratory were major concerns that affected the total TAT of the specimen workflow in the pre-analytical phase. The laboratory can only monitor the department’s pre-analytical activities and are completely incognizant of the status of the collected specimen from other departments until the arrival of the Epic Rover. Using this technology, the laboratory now knows who collected the specimens and at what time but are still vulnerable to delays when multi-tasking ED nurses fail to timely submit the specimens to the laboratory.
Participants
Nurses, physician assistants, medical assistants, and doctors at
the ED joined CPA clerks of the laboratory in the completion of activities
related to the pre-analytical phase. Several HCWs at the ED,
like physician assistants, medical assistants, and doctors, occasionally
helped the nurses with specimen collection and transport. The major
participants of this interdisciplinary field experiment at MSQ were
a) The ED providers, who initiated the specimen workflow by
electronically submitting the appropriate orders in HIS,
b) ED nurses, who collected the specimens then transported
the specimens to the laboratory on time, and
c) CPA clerks, who processed the specimens for laboratory
testing by the CLT.
MSQ ED nurses worked 12-hour shifts from 7 AM to 7 PM (Day shift) and 7 PM to 7 AM (Night shift) while CPA clerks worked 7.5- hour shifts from 7 AM to 2:30 PM (day shift), 4 PM to 11:30 PM (Evening shift), and 11:30 PM to 7 AM (Night shift). There was another CPA clerk who worked from 8:30 AM to 4 PM to keep pace with the higher workload and to ensure a seamless transition between day and evening shifts.
The Conceptual Model
The Systems Engineering Initiative for Patient Safety (SEIPS) model is the conceptual model used for this PIP to identify the common sources of delays in the work system of laboratory testing. We used the SEIPS model approach to evaluate and improve the specimen workflow processes to incorporate the pre-analytical activities outside the laboratory. The SEIPS model has six elements, and the interactions between interdepartmental HCWs represented the activities in the external environment illustrated in Figure 3. The first element is the person, which includes the patient, treatment team, and allied health professionals such as medical assistants, phlebotomists, and respiratory therapists. Tasks are the second element, which are activities related to the collection processes. Technologies and tools are the third element, which includes the Epic Rover collection documentation device, HIS and LIS computer software, flatscreen, and Vocera cell phones. The organization is the fourth element, which provides for institutional culture, protocols, and managerial approaches. The physical environment is the fifth element, which includes structural design and surrounding necessities, such as lights, ventilation, noise control, and privacy. The external environment is the sixth element, which provides delivery of care and the reporting system. The work system activities or external environment played a significant role in initiating the testing process to achieve favorable patient outcomes (Figure 4).
Outcome Measures
This field experiment was designed to identify the common sources of delays in specimen delivery from ED to the laboratory. The following outcome measures were collected:
1) Number of laboratory interventions: Number of laboratory
interventions represented the number of phone calls made by
CPA clerks to the collector of specimens that were not submitted
to the lab after 21 minutes of collection to inquire about the submission
delay.
2) Outcome of specimens: Outcome of specimens represented
the specimens’ final status that was not submitted to the lab after
21 minutes of collection. Outcome of the specimens was a categorical
variable with two levels: received (delayed specimen received
after the intervention) vs. canceled (no specimens received
prompting the lab to cancel).
3) Reasons for the specimens that were not submitted to the
lab after 21 minutes of the collection were also collected.
4) TAT (in minutes) from intervention call to the ED collector
to the time the laboratory received the specimens.
In addition to the outcomes, information of work shift, when the
submission delay occurred, was also obtained from a respective set of
HCW rosters in the day and night shift.
Statistical Analysis
Data were entered into and analyzed using SPSS version 23 for Windows (IBM Corp., Armonk, NY). Number of laboratory interventions (number of phone calls made by CPA clerks to the collector of specimens that were not submitted to the lab after 21 minutes of collection to inquire about the submission delay) was derived from the PIP log and divided into two groups based on the outcome of specimens (received vs. canceled). Frequency tables were used to summarize
a) Outcome of specimens by work shift, and
b) Causes of submission delay.
Descriptive statistics were used to summarize TAT (in minutes) from intervention call to the ED collector to the time the laboratory received the specimens. A chi-square test of independence (Field [21]) was used to determine if there was an association between work shift and outcome of specimens. To ensure the validity of the analysis results, we examined the following two assumptions on the Chi-square test
a) Independence of observations, and
b) All cells should have expected counts greater than five (Field
[21]).
Both assumptions were satisfied. The collected data were from two different intervention outcomes (Cancelled vs. Received), and hence it was reasonable to assume observations were independent. A p-value of less than 0.05 indicated significance.
The four-month field experiment generated a total of 133 laboratory interventions (calls made by CPA clerks to the collector of specimens that were not submitted to the lab after 21 minutes of collection to inquire about the submission delay) that involved 60 nurses. Table 1 showed the frequency table of the outcome of specimens by work shift. Of the 133 specimens that required laboratory interventions, 82 (61.7%) were submitted after the CPA clerks called the collector of specimens to inquire about the submission delay and 51 (38.3%) were canceled. Furthermore, of the 133 laboratory interventions, 105 (78.9%) occurred during the day shifts, and 28 (21.1%) occurred during the night shifts. The results of the chi-square test of independence indicated that there was no statistically significant association between shift and outcome of specimens (χ₂ (N = 133, 1) = 0.104, p = 0.747). Several unavoidable causes of delays were identified, including difficult veins, patients unable to void urine, malfunctioning Epic Rover devices, and unavailability of patients due to procedures at other ancillary services such as Radiology and Dialysis. Notably, of the 133 specimens that required laboratory interventions, nearly half of them (46.6%) were not given specific reasons for the delay (Table 2).
Note: CLT = Clinical laboratory technologist; CPA = Central processing area; ED = Emergency department; HIS = Hospital information system; LIS = Laboratory information system; TAT = turnaround time.
Note: Outcome of specimens: received (delayed specimen received after the intervention) vs. canceled (no specimens received prompting the lab to cancel). Others = included Epic Rover printer issues encountered by ED nurses and CPA workload issues.
The unspecified reasons for the delay may indicate that the ED collectors were distracted after collecting the specimens during the four-month field experiment. The study also found that 14 of the outliers (10.5%) were unavoidable due to difficult collection; 4.5% were caused by the combination of the challenges faced by nurses with Epic Rover (N = 4) and overwhelming workflow faced by CPA clerks (N = 2) (Table 2). The specimens received after difficult collections had the highest mean TAT in minutes from intervention call to the ED collector to the time the laboratory received the specimens (M = 119.0, SD = 132.8), followed by the other specimens that involved barcode label printer issues in ED combined with workload issues at the laboratory (M = 32.0, SD = 18.2), and finally, the specimens received without explanation (M=19.6, SD = 26.0) as detailed in Table 3. We also determined that the laboratory received overdue specimens within 20 minutes after calling the collector in over half (58.5%) of the total number of received specimens (Table 4). Table 4 also showed that 61% of specimens received after intervention were received over one hour from the collection time, potentially generating inaccurate lab results (Table 5).
Table 3: Summary Statistics of TAT in Minutes from Intervention Call to the ED Collector to the Time the Laboratory Received the Specimens.
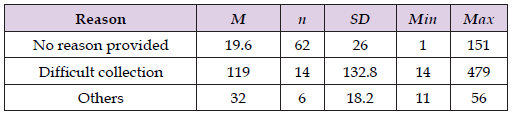
Note: The lab monitored the time it took for the ED nurse to send the collected specimens after calling (intervention).
Note: Outcome of specimens: received (delayed specimen received after the intervention) vs. canceled (no specimens received prompting the lab to cancel).
The common reason for the missing collection information is when the collector fails to document in the HIS either through the application of Epic Rover or from a computer on the nursing station or workstation on wheels (WOW). For example, Epic Rover users may fail to follow step four of the Epic Tip Sheet for PCAs (Figure 6). HCWs who chose not to use the Epic Rover can still document the collection information by following step 3 of the Epic Tip Sheet in Hyperspace (Figure 7). The Epic Rover, which replaced an older system called Data Fusion on November 27, 2017, is a major device technological upgrade that transmits specimen collection information to HIS. The early stages of Epic Rover implementation were when several HCWs submitted the specimens to the lab using the downtime paper requisitions without barcoded labels on the specimens instead of the expected electronic transmission of collection information from a HIS to LIS. CPA clerks had to either manually order the tests and manually input the collection information if such information is available in the requisition.
The second limitation of the study was the staffing issue in the laboratory. In addition to monitor the process of specimen collection, CPA clerks also had to respond to other critical areas like the operating room, ICU, and chemotherapy departments. Staffing issues in the laboratory limited the ability to call the ED nurse who failed to submit collected samples to the laboratory in a timely fashion. Working understaffed limited the sample size down to 133 interventions. The study’s third limitation was that Vocera phones may mal-function and the pneumatic tube system may occasionally break down. Mal-functioned Vocera phones prevented instant connection between the CPA clerks to the ED nurse who collected the specimens that were not submitted within the required timeframe. The pneumatic tube system’s occasional breakdown caused specimen transport delays because the ED staff had to hand-deliver the specimens. ED HCWs batched the specimens before sending to the lab instead of hand delivering every sample after collection. The fourth and final limitation of the study was the amount of time for the field experiment, which was only four months from August to November of 2018. This limitation exists due to a doctoral program’s time constraints and very difficult to overcome unless a researcher devotes personal time for future research. Data from different seasons of the year would provide a better understanding if a particular pattern exists on the different reasons for delays in specimen delivery.
The study showed that distracted HCWs from the ED were the leading causes of delays between specimen collection and specimen delivery to the laboratory. More importantly, the study proved that the laboratory interventions helped decrease the TAT from specimen collection to specimen received, which helped move the average time from patient arrival to provider disposition. The methodology implemented to monitor all collected samples also served as a safety net that the laboratory can use for other departments such as clinics and nursing stations that routinely faced the risk-of leaving the collected specimens behind and exceeding the stability period before delivering to the laboratory. Maximizing the potential of technological devices such as the Epic Rover and the Vocera phones improves the TAT in the pre-analytical phase and enhances the professional relationships between ED and laboratory team members. We also concluded that all HCWs faced the same types of distractions and patient conditions that cause delays regardless of the time of day and the set of the roster on duty.


