Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Mbongue-Mikangue CA1, Saké Ngané CS1, Njiki-Bikoï J1, Membangbi AE1, Mekounthé-Motso M1, Mbaga DS1, Soh LB1, Mahoumo Fodop A1, Kwedjeu CS1, Touangnou-Chamba SA1, Njiki-Bikoï AU2 and Riwom Essama SH1
Received: January 03, 2024; Published: March 25, 2024
*Corresponding author: Mbongue Mikangue CA, The University of Yaounde I, Faculty of Sciences, Department of Microbiology, Cameroon
DOI: 10.26717/BJSTR.2024.55.008751
Background: Cameroon is a country located in sub-Saharan Africa, area Endemic to the Herpesviridae Family, there is Very Little Data on the Herpes Virus Infections Epidemiology, Especially Associated with HIV Infection with HIV-positive people born HIV positive with an undetectable viral load. The aim of our study was to determine the seroprevalence of four herpes viruses: cytomegalovirus (CMV), Epstein-Barr virus (EBV), Herpes Simplex virus 1 (HSV-1) and 2 (HSV-2) in HIV-positive children born HIV positive in Yaoundé.
Methods: It was a prospective cross-sectional study, conducted at the YUTH after 12 months of follow up, in children living with HIV born HIV positive, on antiretroviral treatment and whose medical file was complete and available within the ATC. IgG/IgM antibodies against HSV-1, HSV-2, CMV, and IgM against EBV were qualitatively determined by Rapid Diagnostic Tests, for the detection of these pathogens. Data entry and analysis was done using the Statistical Package for Social Sciences (SPSS) version 22.0, the Fischer exact, the Khi-square and the Mann–Whitney tests. P-values less than 0.05 were considered statistically significant.
Results: 74 participants were enrolled in the study with a female predominance of 68.92% (n=51/74). The average age of our series was 9.05±5.09 years, and most participants was under 10 years old (56.76%, n=42/74). CMV, HSV-1, HSV-2 and EBV Seroprevalences were 95.95%, 93.24 %, 93.24 %, 22.97 % respectively. The most spread co-infection was that of HIV-1/CMV/HSV-1/ HSV-2 43 participants (63.51%). Other parameters such as sex, age, stage of disease, smoking and alcohol consumption were significantly associated with the seropositivity of these herpesviridae.
Conclusion: Despite the absence for most of the clinical manifestations related to CMV, HSV-1 and HSV-2, it was strong to note a high circulation of those viruses in HIV infected patients, mainly in bi and tri co-infections.
Keywords: Children; Herpesvirus; HIV; Seroprevalence
Abbreviations: HIV: Human Immunodeficiency Virus; EBV: Epstein-Barr Virus; HSV: Herpes Simplex Virus; CMV: Cytomegalovirus; SPSS: Statistical Package for Social Sciences; NACC: National AIDS Control Committee; KS: Kaposi Sarcoma; YUTH: Yaounde University Teaching Hospital; ATC: Approved Treatment Center; HHV: Human Herpesvirus
Since its discovery, Human Immunodeficiency Virus (HIV) infection remains a real health problem, and particularly in sub-Saharan Africa, and according to the data of the National AIDS Control Committee (NACC), Cameroon in 2021, the prevalence of HIV infection was 3.1% [1]. HIV infection promotes exogenous infections or reactivation of solely controlled infections, with the LT CD4+ lynphopenia. With the weakening of the immune system, the reactivations concern more tuberculosis and the oncogenic viruses such as herpes viruses [2]. Herpesviridae are among the few known causes of cancer and contribute to a variety of malignancies worldwide. The agents considered here, termed Epstein-Barr virus (EBV) and Kaposi sarcoma herpesvirus (KSHV–human herpesvirus 8 [HHV8]), are members of the gamma-herpesvirus subfamily [3]. And are a family of managerial viruses such as Kaposi Sarcoma and lymphomas, opportunistic infections of HIV/AIDS [4]. The Herpes simplex virus (HSV), responsible for a contagious disease affecting the skin and mucous membranes, is characterized by a vesicular eruption of grouped pimples [5].
A disease long considered begining in immunocompetent subjects; it can be very severe in subjects with immunodeficiency. [6,7]. The Epstein-Barr virus (EBV) and cytomegalovirus (CMV) is common in HIV-infected individuals. Pathogenic gene products enhance the replication of HIV by transactivation of HIV long terminal repeats (LTRs) and are involved in infectious mononucleosis and associated lymphomas, while the type 8 human herpes virus (HHV8) enters in the occurrence of Kaposi sarcoma (KS) and associated lymphomas. The immunosuppression of the individual, obesity, excessive alcohol, and smoking considers as risk factors for the emergence of these viruses, [8-10]. Although Cameroon is an endemic zone for human herpes virus, and the work done by Njiki et al., In 2015 showing that there is very little information on the epidemiology of HHV8 infection particularly that associated with HIV infection [11], the finding remains with other viruses of the same family. The aim of this study was to determine the seroprevalence of four viruses HSV-1, HSV-2, EBV and CMV in patients born HIV positive at the Yaounde University Teaching Hospital (YUTH).
Study Design and Setting
It was a prospective cross-sectional study, conducted from November 2020 to October 2021, in YUTH, on patients who came for consultation or were followed at the Approved Treatment Center (ATC).
Participants Enrolment
For every participant who completed the inclusion criteria, a written informed consent was obtained from the parents, and a technical sheet had to be filled by each participant, providing the socio-demographic and clinical status as age sex and HIV infection. These data were completed and/or confirmed by the patient’s medical record.
Laboratory Methods
Then a sampling of venous blood into an EDTA tube of 5ml was made and the samples taken then transported to the Microbiology Laboratory of the Faculty of Science. At the laboratory, after centrifugation (at 1300 rpm for 10 min, between 18-24 °C), the plasma obtained was kept in a freezer at -25 °C, for the later serological research of the infectious agents [12]. According to the manufacturer’s instructions, the kits and samples to be tested were first brought to room temperature. For the detection of IgM and IgG antibodies directed against CMV, HSV-1 and HSV-2, the One Step TORCH IgM/IgG kit (TOX IgM/IgG, RV IgM/IgG, CMV IgM/IgG, HSV-I /2IgM/IgG (Bioneavan co.LTD., NO.18 Ke YuanLu, GongYeKaiFaQu, Huang Cun Zhen AaXing County, Beijing) was used, and for the detection of IgM antibodies directed against EBV, the Epstein Barr Rapid Diagnostic kit (EB) -IgM antibody was used according to the kit manufacturer’s instructions (Bioneavan co.LTD., Beijing). The test card was placed on a dry horizontal work surface, then 30μl of sample plasma added on top. When the sample migration was found to be difficult, 20μl of sample dilution solution was added immediately, and an additional 50μl of the same solution 5 minutes later. 15 to 20 minutes after the addition of the plasma, the different results were observed [13].
Data Management and Analysis
For each participant, data on parameters of interest gathered through interviews and by blood analysis were recorded and processed using Excel 2016, and the statistical analysis was done using IBM Statistical Package for Social Science Version 22.0. On one hand, we used the Fischer exact and the Chi-square tests to compare qualitative variables between groups, while on the other hand, we used the Mann–Whitney test to compare quantitative variables. All p values below 0.05 were considered significant.
Ethical Considerations
The study was approved by the Regional Ethics Committee for Research in Human Health (N°0082/CRERSHC/2023) and received authorization from the Yaounde University and Teaching Hospital (N°494/AR/CHUY/DG/DGA/CAPRC). The Microbiology Laboratory allowed the laboratory analyses. Patients signed consent forms regarding the use of their plasma and the collection of their medical data.
Sociodemographic and Clinical Parameters
The average age in the study was 9.05±5.09 years, the children were in majority (56.76%, n=42/74) under 10 years old and of female gender (68.92%, n=51/74). 18/74 children (24.32%) smoked and 17/74 concerned by alcohol consumption (22.97%). HIV-1 infection was the most encountered in our cohort, with 94.60% of participants at WHO stage I of the disease. According to medical records, the most commonly used protocol was Tenofovir-Lamivudine-Efavirenz (TDF/3TC/EFV) with 79.73% of participants (Table 1).
Seroprevalence of Herpesviridae
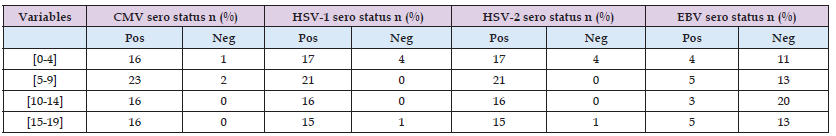
The seroprevalence of CMV was 95.95% (n=71/74), that of HSV-1 at 93.24% (n=69/74), that of HSV-2 at 93.24% (n=69/74) that of EBV was 22.97% (n=17/74). Seropositivity for HSV-2, HSV-1 and CMV was statically associated with HIV infection (p<0.05) (Table 2). Our participants presented multi-infections to herpesviridae. Most co-infections found was CMV/HSV-1/HSV-2 63.51%, (n=43) (Table 3). The co-infections found affected all age groups (Table 3). Ages ranging from 10 to 19 years concentrate the greatest number of all the consumption of alcohol (n=25/74) and tobacco (n=18/74) (Table 4) and Ages ranging from 0 to 14 years concentrate the greatest number of co-infections (Table 5).
Table 2: Distribution of participants according to IgG/IgM antibodies against HSV-1, HSV-2, CMV, and IgM against EBV results.
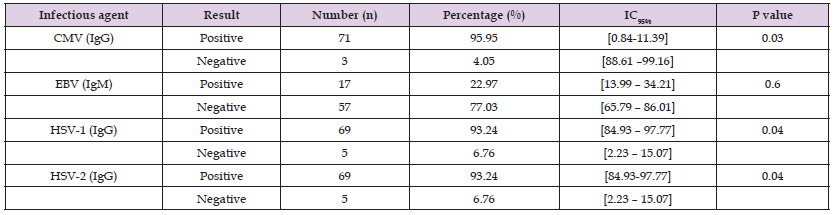
Note: HSV-1/-2: Herpes simplex Virus-1/-2. HIV-1: Human immunodeficiency virus type 1. HIV-2: Human immunodeficiency virus type 2. CMV: Cytomegalovirus. EBV: Epstein-Barr virus, RV: Rubella virus.
Table 4: Frequency of HIV infection according to age group and alcohol, Tobacco, Alcohol and Tobacco consumption.
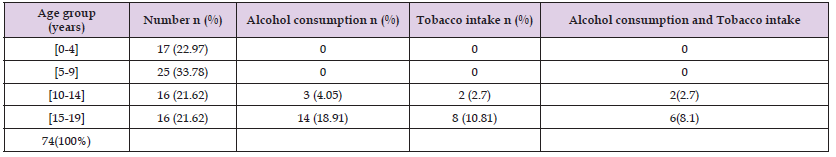
Table 5: Frequency of herpesviridae infection according to age group and alcohol, Tobacco, Alcohol and Tobacco consumption.
Epidemiological data for herpesviridae in Cameroon is not well documented [11]. Among the opportunistic infections of viral origin, infections caused by Herpesviridae, are often responsible for a contagious disease affecting the skin and mucous membranes, is characterized by a vesicular eruption of grouped pimples but also cancers [14]. Both human immunodeficiency virus (HIV) and human herpesvirus (HHV) infections persist lifelong, and almost all individuals infected with HIV are also infected with ≥1 HHV [15]. Our study revealed a female predominance (68.92%, n=51/74) with an average age of 9.05±5.09 years, the majority of participants were under 10 years. A study conducted by Njimbam et al., at the Cité Verte Subdivisional Medical Center of Yaoundé in 2016, reported 66% of women [16]. This observation corroborates the feminization of the HIV pandemic, which is a major trend. Currently 50% of people living with HIV in the world are women, with this rate reaching 59% in sub-Saharan Africa, the region most affected by the epidemic. Certainly, the risk of contamination during sexual intercourse is greater among them, but they are also in a situation of greater social and economic vulnerability, and therefore greater exposure to risks, particularly in relation to AIDS [17].
The age groups obtained during our study follow the concerned population trend of PLHIV in Cameroon [18]. Most of our participants were under TDF/3TC/EFV treatment protocol (79.73%), a first-line regimen. This protocol is a preferred option in countries with limited resources, because it is simple, inexpensive, in combined form and can be used in HIV/HBV, HIV/BK and in pregnant and breastfeeding women [1]. In our study, IgG seroprevalences were 95.95% for CMV, 93.24% for HSV-1, 93.24% for HSV-2, IgM seroprevalence was 22.97% for EBV. This high antibody seroprevalence demonstrates the widespread circulation of these viruses in the population, confirming the status of an endemic area for herpes viruses [14]. Also, to date, the population changes its sexual practices, such as oral sex associated with sociocultural changes, may explain the increasing trend of these infections [11]. The majority of co-infections between herpesviridae found concerned HSV-1 and HSV-2 with a prevalence of 47(63.51) this result is high compared to that reported by Njimbam et al (2016) which was 34% [16]. It is becoming urgent to sensitize young people born HIV positive because the HIV-HSV interaction would be the cause of several resistances to antiretrovirals with the formation of pseudotyped viruses (i.e. viral particles comprising the HIV genome enveloped in surface glycoproteins derived from other viruses) [19,20].
EBV and CMV co-infections appear to be more frequent in the literature. A possible explanation is that most of the patients in this cohort, although hospitalized, were seen in outpatient care [21]. Testing for the presence of CMV infection is particularly important for HIV-positive patients to assess disease severity and monitor response to treatment. The influence of factors not investigated in this study such as genetics, nutritional status, socio-economic conditions could explain these a priori weight results concerning the prevalence of CMV [22]. Ages ranging from 0 to 15 years concentrate the greatest number of co-infections, and ages ranging from 10 to 19 yers concentrate the risk factors for the occurrence of these co-infections (alcohol and tobacco consumption). If alcohol acts as a behavioral risk factor in acquisition of HIV, it also acts at the biological level through its immunosuppressive role increasing susceptibility to infections by reducing the inflammatory response [23], thus finding the smoking which is also associated with the metabolic complications of certain antiretrovirals [4]. These data underline the need to implement preventive actions, particularly with regard to the consumption of alcohol and increased dietary support when stopping smoking [24]. The present study confirmed that herpesviridae prevalence among children born hiv positive at the yaounde university teaching hospital, Cameroon after 12 months of follow-up was high and this might increase risk of cancers. Our study has strengths including that it was carried out on children who were born HIV positive to HIV-positive mothers and had an undetectable viral load; the study provided information on the epidemiology of herpesviridae in young people. HIV infected born HIV positive. Furthermore, our study has limitations due to its monocentric and transversal nature. The absence of molecular analyzes would make it possible to confirm infections or even co-infections.
Our study aimed to determine the seroprevalence of herpesviridae in children born HIV with an undetectable viral load at the Hospital and University Center of Yaounde. Our study allowed us to evaluate the co-infection of four herpesviridae (EBV, CMV, HSV-1 and 2) in children born HIV positive at the YUTH. This study highlighted a high risk of herpesvirus infections among PLHIV born HIV positive, in ages ranging from 3 to 19 years, especially among women. The seroprevalences of these viruses were high and their association with children clinical profile highlights their risk factors. HSV-1, HSV-2 and CMV seroprevalences were higher in coinfection than in monoinfection. The prevalence of these viruses demonstrates their high endemicity in Cameroon.
The authors declare no conflict of interest.
Riwom E.S. Honorine, Njiki B. Jacky designed and set up the research project. Mbongué M.C. André, collected the samples, with Njiki B. Jacky and Mbaga D. Serge, led the technical aspects at the Microbiology Laboratory. The analysis of the data and the writing of this article saw the collaboration of all authors.
The authors would like to acknowledge all the participants who contributed to this research. The authors would also like to acknowledge the staff of the Yaoundé University Teaching Hospital, Cameroon.
The authors received no funding support for the research, authorship, and publication of this article.
The data supporting the results of this study are available on request from the corresponding author. The data is not publicly available because it contains information that could compromise the confidentiality of research participants.


