Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Mourad Belkhelfa1*, Narimene Beder1, Rama Touahri2, Chafia Toui Boukoffa1 and Abdelbassat Ketfi2
Received: March 18, 2024; Published: March 25, 2024
*Corresponding author: Mourad Belkhelfa, Cytokines and NO-Synthases, Immunity and Pathogeny Team, Laboratory of Cellular and Molecular Biology, Faculty of Biological Science, University of sciences and technology Houari Boumediene, Algiers, Algeria
DOI: 10.26717/BJSTR.2024.55.008749
Background: Coronavirus disease 2019, the highly contagious infectious disease caused by severe acute respiratory syndrome coronavirus 2. Coronavirus uses angiotensin-converting enzyme-2, which is highly expressed in the human lower respiratory tract but also in other tissues, as the cellular entry receptor. Thus, COVID-19 mainly affects the respiratory system, but can cause damage to other body systems, including the cardiovascular, gastrointestinal, hepatobiliary, renal, and central nervous systems.
Methods: In the first step of our study, we evaluated the activity of total lactate dehydrogenase in the different groups of body mass index patients associated or not with pathologies (hypertension and cardiovascular disease, diabetes). In the second step, we have evaluated the therapeutic efficacy by measurement of the total lactate dehydrogenase-1 activity before treatment and total lactate dehydrogenase-2 activity after treatment in patients associated or not with pathologies.
Results: Interestingly, we reported that the association of cardiovascular disease, hypertension and diabetes with obesity does not allow the reduction of total lactate dehydrogenase activity. Nevertheless, in the groups of patients with hypertension and cardiovascular diseases, the treatment doesn’t reduce the total lactate dehydrogenase activity, and preserves the effectiveness of treatment.
Conclusions: Our results suggest that associated obesity with cardiovascular diseases, hypertension and diabetes affect the effectiveness of treatments in patients with COVID-19 infection.
Keywords: COVID-19; Lactate Dehydrogenase; Diabetes; Cardiovascular Disease; Hypertension
Abbreviations: COVID-19: Coronavirus Disease; ACE2: Angiotensin-Converting Enzyme 2; tLDH: Total Lactate Dehydrogenase; HT: Hypertension; AZM: Azithromycin; BMI: Body Mass Index
Coronavirus disease 2019 (COVID-19), is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. The clinical presentation of COVID-19 varies widely, ranging from asymptomatic to mild or severe forms. In the early stages of the disease, most patients experience mild clinical symptoms, including a high fever and dry cough. However, 20% of patients rapidly progress to severe illness characterized by atypical interstitial bilateral pneumonia, acute respiratory distress syndrome and multiorgan dysfunction [1]. The primary cell attachment factor in SARS-CoV-2 has been confirmed to be the human angiotensin-converting enzyme 2 (ACE2) cell surface receptor [2]. This single transmembrane domain protein is expressed at appreciable protein levels on terminal bronchiole as well as type I and II lung alveolar epithelial cells, which aligns with the primary pathology of COVID-19: loss of oxygen saturation due to poor pulmonary gas exchange. Likewise, noteworthy ACE2 levels have been reported among enterocytes of the small intestine, arterial, and venous endothelial cells, and arterial smooth muscle cells in most organs, as well as the vagus nerve innervating the lung, heart and digestive system [3], which mirror the diversity of symptoms and pathologies associated with COVID-19: diarrhea, myocarditis, fatigue, encephalopathy (headaches, confusion, anosmia, stroke-like symptoms, and seizures) [4].
In our current study, we are focusing on total lactate dehydrogenase (tLDH) assessment. tLDH is an interesting biomarker for monitoring the course of patients infected with COVID-19 because high levels of tLDH have been associated with worse outcomes in patients with other viral infections in the past [5]. LDH is an intracellular enzyme found in cells in almost all organ systems, which catalyzes the interconversion of pyruvate and lactate, with concomitant interconversion of NADH and NAD+ [6]. It is present in humans in five separate isozymes: LDH-1 in cardiomyocytes, LDH-2 in the reticuloendothelial system, LDH-3 in pneumocytes, LDH-4 in the kidneys and pancreas, and LDH-5 in liver and striated muscle. Although LDH has been traditionally used as a marker of cardiac damage, abnormal values can result from multiple organ injury and decreased oxygenation with up regulation of the glycolytic pathway [7]. Thus, COVID-19 mainly affects the respiratory system, but can cause damage to other body systems, including the cardiovascular, gastrointestinal, hepatobiliary, renal, and central nervous systems [8]. The common comorbidity in patients with COVID-19 are hypertension (HT), cardiovascular disease, diabetes, obesity, and respiratory disease. The use of drugs can restore, strengthen, and modulate the immune system is, therefore, a perfect approach to handling the COVID-19 infection. In this way, we talk about some drugs such as hydroxychloroquine (HCQ), and Azithromycin® (AZM). Several in vitro studies showed a possible antiviral and anti-inflammatory effect of HCQ and AZM in preclinical models of viral infections [9]. HCQ induces the production of reactive oxygen species (ROS) mediated toxicity in inner glial cells after prolonged treatment requiring the use of vitamin C to prevent the ROS production [10].
Furthermore, vitamin C reduces pulmonary lung inflammation and lung injury due to ROS release by phagocytes [11], the reason why treatment was effective in the reduction of tLDH activity levels. We also administered AZM, which inhibits the synthesis of bacterial proteins [12]. Recently, AZM has received increasing attention because of additional properties on a host-defense reactions by exerting the immunomodulation effects in chronic inflammatory diseases [12]. The modulation of immune responses enables the lasting therapeutic advantage of AZM in chronic obstructive pulmonary disease [12]. This seems interesting because when the immune system is weakened by the SARS-Cov-2 infection, bacterial infections will take place. For this reason, we opted for this anti-biotherapy for the improvement of treatment protocol administered to patients in the state of co-infection, so this type of infection could be prevented to avoid the deterioration of other organs. We added also in treatment protocol-2 Cefotaxime® which has a broad antibacterial spectrum and is active against Gram-positive bacteria [13], Teicoplanine® which has antiviral activity against influenza A and B viruses, composed by lipoglycopeptides: bioactive molecules against human coronavirus [14]. The first objective of our study is to investigate the eventual correlation between the total LDH activity levels: enzymatic biomarkers, measured in plasma of patients COVID-19 infection, with different categories of body mass index (BMI) patient’s. Secondly, we highlight the important impact of associated pathologies: HT, cardiovascular disease and diabetes on the tLDH activity levels before and after treatments. By this way we aimed to elucidate also the effectiveness of the two therapeutic protocols which are adopted according to the severity of the viral infection in relation with tissue damage which can be demonstrated by tLDH activity level reduction.
The pandemic health situation drew our attention as researchers and clinicians, thereby we have tried to find durable solutions both at preventive and treatment levels. This work has been carried out in accordance with the code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans, consent was obtained for experimentation with human subjects, the privacy rights of human subjects must always be observed. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. In our study, we opted for the assay of total LDH activity level in patients with COVID-19 infection before and after treatment, while following two different treatment therapeutic protocols. The therapeutic protocol 1 (Protocol-1) consist of the use: Hydroxychloroquine (HCQ), Azithromycin® (AZM), Vitamin C, Zinc, Preventive Enoxaparin®, and in therapeutic protocol 2 (Protocol-2): we use the same therapeutic approach 1 with the use of a curative dose of Enoxaparin®, Cefotaxime®, Teicoplanine®, Corticosteroid therapy and Oxygen therapy, according to the severity of COVID-19 infection. We note that the duration of treatment is variable depending on the evolution of the patient's state of health.
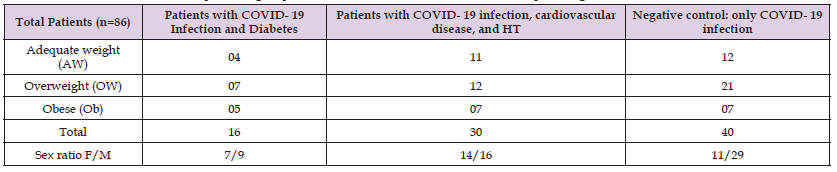
In total we have 86 patients (Table 1), using 1-Biolabo 240T x and Biolisis 50i (Spinreact®), the standard value of tLDH is 200 to 400 IU/L (Pyruvate lactate assay). After receiving the patients who suffered from this viral infection, first we examined the tLDH level from a group of patients before any treatment protocol (tLDH-1), then gave the treatment protocol-1 during the hospitalization, these patients all remained under medical supervision until the end of treatment. In the second step we examined the tLDH level after treatment (tLDH-2). The therapeutic protocol-1 was modified to obtain a second therapeutic protocol-2 by adding of three drugs (Cefotaxime®, Teicoplanin® and Corticosteroid therapy) associated with oxygen therapy in order to reduce the intensity of the various symptoms due to the COVID-19 infection and improve the state of health of the patients [15]. In the therapeutic protocol-2 we use the same procedure as the first treatment protocol-1: we evaluate the tLDH activity level in the second group of patients before treatment protocol-2 (tLDH-1) and after treatment (tLDH-2). However, patients suffering from several other pathologies which are at the origin of the worsening of their state of health are excluded from this study.
Table 1: Stratification of different patients groups with COVID-19 infection and associated pathologies.
Statistical Analysis
In this study we used the ANOVA test for statistical analysis.
Comparison between Total LDH Activity Levels Measured Before and After Using the Two Protocols Treatment in Patients COVID-19 Infection
We note with interest a global down regulation in tLDH-2 activity levels as shown in comparison with the tLDH-1 activity rate, this result is the same that obtained in both protocols followed. Protocol-1: Average tLDH-1 activity level (514±277 UI/L), Average tLDH-2 activity level (359±216 UI/L), and Protocol-2 Average tLDH-1 activity level (687±349 UI/L), Average tLDH-2 activity level (450±285 UI/L).
Relationship between COVID-19 Infection, Cardiovascular Disease, Hypertension (HT) and tLDH Activity Level
In the first step of our study, we investigated the influence of variation BMI on tLDH activity level in patients with cardiovascular disease and HT.
Effect of Cardiovascular Disease and Hypertension on tLDH Activity Level in Different BMI Groups of Patients: As shown in (Figure 1) the analysis of tLDH activity level measured in plasma of patients with COVID-19 infection, associated with HT and cardiovascular disease in different BMI groups (AW: 456±177 UI/L, OW; 562±392 UI/L and Ob: 601±454 UI/L) showed no significant difference (p>0.05) compared to the negative control groups (AW: 472 ± 211 UI/L, OW: 584 ± 312 UI/L and Ob: 509 ± 153 UI/L). We noted with interest that HT and cardiovascular disease and/or obesity did not modulate the activity of tLDH-1 during the infection with COVID-19 before any treatment (Figure 1).
Effect of the Therapeutic Protocol on the tLDH Activity Levels in different BMI Groups of Patients Associate with Cardiovascular Disease and HT: Considering the variation in tLDH activity levels measured in plasma of different BMI groups of patients with COVID-19 infection, and associated with cardiovascular disease and HT shown in (Figure 2) is revealed significantly decreased activity level of tLDH-2 (p<0.05) in negative control group of AW, that we noted effective response to the treatment protocols, AW (tLDH-1: 472±211 UI/L and tLDH-2: 331±128 UI/L). However, in the other groups we observed that cardiovascular disease and obesity suppress the effect of treatment on tLDH activity level (Figure 2). The cardiovascular disease and HT associated with COVID-19 infection revealed no significant difference (p>0.05) between tLDH-1 (AW: 472±211 UI/L, OW: 584±312 UI/L and Ob: 601±454 UI/L) and tLDH-2 (AW: 409±99 UI/L, OW: 378±108 UI/L and Ob: 493±380 UI/L).The obesity associated with COVID-19 infection (negative control) revealed no significant difference (p>0.05) between tLDH-1 (OW: 584±312 UI/L and Ob: 503±171 UI/L) and tLDH-2 (OW: 426±184 UI/L and Ob: 427±114 UI/L).
Relationship between COVID-19 Infection and Diabetes and tLDH Activity Level
In the second step of our study, we investigated the influence of BMI variation on tLDH activity levels in patients with diabetes.
Effect of Diabetes on a tLDH-1 Activity Level in different BMI Groups: As shown in (Figure 3) the analysis of the tLDH-1 activity level measured in plasma of patients with COVID-19 infection, associated with diabetes in different BMI groups (AW: 427±104 UI/L, OW: 457±123 UI/L, and Ob: 653±512 UI/L) are revealed not any significant difference (p>0.05) compared to the groups of negative control subjects (AW: 472±211 UI/L, OW: 585±315 UI/L, and Ob: 503±171 UI/L). We noted that diabetes and/or obesity did not modulate the activity levels of tLDH-1 in the event of infection with COVID-19 (Figure 3).
The Effect of the Therapeutic Protocol on tLDH Activity Levels in Different BMI Groups of Patients with Diabetes: We observed in (Figure 4) that there is a significant decreased activity level of tLDH-2 (P<0.05) measured in the AW group negative control, that we noted effective response to the treatment protocols, AW (tLDH-1: 427±104 UI/L and tLDH-2: 409±87 UI/L). However, in the other groups we observed that diabetes and obesity suppress the effect of treatment on tLDH activity level (Figure 4). The diabetes associated with COVID-19 infection revealed no significant difference between tLDH-1 (AW: 427±104 UI/L, OW: 457±123 UI/L and Ob: 653±512 UI/L) and tLDH-2 (AW: 409±87 UI/L, OW: 385±87 UI/L and Ob: 287±184 UI/L). The obesity associated with COVID-19 infection (negative control) revealed no significant difference between tLDH-1 (OW: 584±312 UI/L and Ob: 503±171 UI/L) and tLDH-2 (OW: 426±184 UI/L and Ob: 427±114 UI/L).
We observed during the analysis of our data that the activity levels of tLDH is upregulated during viral infection by COVID-19, this observation is confirmed by the systemic elevated concentrations of tLDH during diagnosis upstream of treatment compared with the standard value (200-400 UI/L) in different groups of BMI (AW, OW and Ob). Our results indicate that the use of treatment protocols is effective. There is a significant difference between tLDH-1 and tLDH-2 demonstrated by ANOVA test. The tLDH activity assay results show that both use treatments protocols (Protocol-1 and Protocol-2) are effective and the obtained results are argued by a down regulation and of tLDH-2 enzyme activity levels in the vast majority of patients who underwent treatment during hospitalization compared to patients whose tLDH-1 values analyzed at the admission of the patients with COVID-19 infection before the treatment. We also noted that the associated pathologies such as hypertension, cardiovascular diseases and diabetes do not modulate the severity of the infection. Contrariwise, they affect the effectiveness of treatments because the patients who suffer from these diseases don’t respond such as negative controls, with the exception of a single case only when the diabetes presents in the obese and overweight groups. We also report that the analysis of tLDH-1 before treatment of patients with COVID-19 infection associated with HT and cardiovascular disease in different BMI groups revealed no significant difference compared to the negative control groups. We concluded that HT and cardiovascular disease modulate the activity of tLDH during infection with COVID-19, and affect negatively the efficacy of treatment protocols. Then we studied the impact of obesity associated with diseases previously cited on the effectiveness of treatment protocols. We noted that diabetes don’t affect the efficacy of treatments in adequate weight subjects with COVID-19 infection. However, we observed in overweight and obese groups that the treatment is less effective.
Our results suggest that associated obesity with cardiovascular diseases, hypertension and diabetes affect the effectiveness of treatments in patients with COVID-19 infection as they are not involved in the severity of viral infection.
The authors thank all patients who are participants in this study for providing blood. They also thank Pr. Houda Belgendouz for her participation in this work.
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The trial was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
All authors have no conflicts of interest to declare.


