Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Hamid Moghaddasi1*, Mohammadreza Naderi Haji2, Babak Sharif Kashani3 and Linda Samadi4
Received: March 18, 2024; Published: March 25, 2024
*Corresponding author: Hamid Moghaddasi, Hamid Moghaddasi (PhD), Professor of Health Information Management and Medical Informatics, Department of Health Information Technology & Management, School of Paramedical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
DOI: 10.26717/BJSTR.2024.55.008748
Between one and two percent of the population of the developed countries are currently treated with oral anticoagulation therapy. The transition of all or part of the responsibility for therapy management to the patient is an appropriate strategy to respond to the increasing demand for oral anticoagulation therapy. The main objective of this original study was to investigate the impact of using mobile applications on health outcomes in patient self-management of oral anticoagulation therapy. An Android mobile application called XrinA was developed to provide warfarin patient self-management. The study was conducted following a Before-After study design. In the “Before” and “After” periods, 21 patients were first treated as usual and then by using the developed application. In the “Before” period, the mean percentage of International Normalized Ratios (INRs) within the therapeutic range and Time in Therapeutic Range (TTR) of patients was 31.5% and 34.3%, respectively. In the “After” period, the mean percentage of INRs within the therapeutic range and TTR of patients was 41.4% and 50.1%. Overall, the use of mHealth applications improved the Patient Self- Management of oral anticoagulation therapy in terms of the percentage of INRs within the therapeutic range and TTR.
Keywords: Vitamin K Antagonists; Oral Anticoagulation Therapy; Self-Management; Mobile Health
Abbreviations: INRs: International Normalized Ratios; IT: Information Technology; mHealth: Mobile Health; NOACs: Novel Oral Anticoagulants; OAT: Oral Anticoagulant Therapy; PSM: Patient Self-Management; TTR: Time in Therapeutic Range; VKAs: Vitamin K Antagonists
Different types of thrombosis and thromboembolic events can be usually prevented and treated using anticoagulants [1]. Oral anticoagulants (VKAs) and newer drugs known as the Novel Oral Anticoagulants (NOACs) [2]. Despite the advent of NOACs, there is limited information on their use among patients with renal dysfunction, obese patients, pediatric population, and pregnant women [2]. Moreover, they cost much more than VKAs [2]. Therefore, VKAs, especially warfarin, line up as the first-choice treatment for a large group of patients [3]. Currently, between one and two percent of the population in the developed countries are estimated to receive oral anticoagulants, especially warfarin, on a regular basis [4]. It should be pointed out that the impact of VKAs depends on receiving appropriate doses [2]. To ensure the effective course of VKA therapy, the Prothrombin Time (PT) test should be performed frequently and at regular intervals in order to determine the extent to which the results lie within the therapeutic range, which is regarded as an important indicator of therapy effectiveness [5-7]. The percentage of Time in Therapeutic Range (TTR) is a measure of the quality of VKA therapy management that can be calculated using various methods. The linear interpolation described by Rosendaal is mostly effective in calculating the percentage of TTR [8,9].
TTR refers to the ratio of the number of total days between the two tests in which the patient’s International Normalized Ratio (INR) lies within the therapeutic range [10,11]. Currently, the growing trend of cardiovascular disease (CVD) and the increase in the population taking oral anticoagulants in the world [12,13] as well as the lack of development of facilities commensurate give rise to more increase in demand than in supply. For instance, if all patients in the UK wanted to attend anticoagulation clinics (ACs) in hospitals, the current number of clinics had to increase five to tenfold [14]. Therefore, developing methods with the capability of continuous monitoring of the patient’s conditions at home, work, travel, or in any circumstance at a lower cost and with the shortest time will be of paramount importance. Studies have shown that the utilization of electronic health (eHealth) applications in patient self-management (PSM) has been effective in chronic diseases [15,16]. These applications serve as a reminder to improve medication adherence and control, assist, and inform patients as well [15]. Utilizing these applications by minimizing or even eliminating the need for clinic visits for routine treatment of some common complications and by facilitating self-management can contribute to the patient’s adherence to therapy and increasing the duration of time in which the patient remains within the target therapeutic range and the percentage of TTR [17].
Given the growing trend of using information technology (IT) in various fields of health as well as the lack of studies on the impact of using mobile health (mHealth) in oral anticoagulation therapy in developing countries, the current study was designed. The main objective of this study was to investigate the impact of using "XrinA" mobile application on health outcomes (the number of results of the INR within the therapeutic range and TTR) in patients’ self-management of oral anticoagulation therapies. However, in this study, warfarin was considered the oral anticoagulant of choice, not novel oral anticoagulants (NOACs).
The Mobile Application Development Process
The demands of application users were identified based on valid texts and experts’ opinions. According to the relevant studies, the functional model of the mobile application for warfarin patient self-management (PSM) was developed, requirements for the mobile application were extracted, and use-case diagrams as well as activity diagrams were drawn. Then, the structural model was developed by extracting classes and drawing Class-Responsibility-Collaboration (CRC) models. The behavioral model was then developed by analyzing the system behavior and drawing the Sequence Diagram. When the class diagram or conceptual model of the application was developed, the application platform and programming language were determined. Eventually, an Android mobile application called XrinA was developed for the purpose of warfarin PSM. The application architecture was designed as a Client-Server model. The application consists of two running parts, including the server part running on the server and the client part running on physicians’ and patients’ phones. The task of the server part is to manage schedules and send reminders to the patient based on the physician’s prescription. Besides, provided that no appropriate feedback is received from the patient about the prescription given, the server part will send out required alerts to the physician. The server part of the application was designed in the Microsoft Visual Studio 2015 and C# language.
The client part, which is a mobile application, was designed in the Android Studio 3 and Java language. The server operating system, the Microsoft Windows Server 2016, and the operating system for clients (i.e., mobile phones) are based on Android. The application can be installed on mobile phones with the Android operating system and Android version 4.2.1 and above. The Microsoft SQL Server 2014 was used to manage the data. Data included patients’ information, schedules associated with each patient, patients’ test results, and the prescriptions given to each patient. The communication between clients and servers was also set up via the Internet and based on the Internet Protocol (IP) of each mobile phone and server. In order to use this application, it is a necessity that users’ mobile phones should be always connected to the Internet and the application should be running (i.e., the users do not log out of the application through the “exit” menu). After coding, the application was tested and then was implemented. To troubleshoot the application described, the three-member research group was provided with the total application process flow and its content. The points of view of each of the mentioned individuals were adopted before and during the application development and then taken into account as well. The application was piloted for two weeks and then finalized.
Study Design
The current study was conducted following a Before-After study design to evaluate the application efficiency.
Sampling Technique
Application users included physicians and patients; therefore, an attempt was made to select them. Among several hospitals and one clinic, the same clinic and a physician who met the inclusion criteria participated the study. Patients referred to the clinic were also selected through purposeful sampling. To do so, all the patients referred to the clinic could take part in the study, provided that they met the inclusion criteria. The inclusion criteria of patients were as follows:
• Participants were selected from patients taking warfarin who referred to the clinic, regardless of their disease.
• Patients whose status was stable.
• Patients who were willing to participate in the project.
• Patients who had a smartphone with the Android operating system.
• Patients who were able to use the mobile application.
• Patients who required warfarin therapy for at least one year.
• The exclusion criteria of patients were as follows:
• Patients with unavailable or incomplete data regarding project implementation.
• Patients who required surgery.
• Patients who developed acute diseases.
• Patients who were unwilling to participate in the project.
According to the above-mentioned criteria, 21 patients were selected from those who referred to the clinic. Patients were interviewed. They were asked about their level of education and how much they adhered to the physician’s instructions in the usual treatment method and followed prescriptions on time.
Evaluation of Application
The application was evaluated in terms of efficiency in maintaining the patients’ INRs within the therapeutic range as well as the patients’ TTR. The current study was conducted following a Before-After study design to evaluate the application efficiency. The required investigations were then performed and the information items required for recording during the study period were determined as well. Eventually, a special medical record form called patients’ medical and laboratory record form was designed to store medical data as well as patients’ INR test results during the two periods of before using the mobile application and after using the mobile application. The form was designed through consulting a two-member research group consisting of a professor of the Department of Health Information Management and Technology of the School of Paramedical Sciences as well as a cardiologist. The medical and laboratory record form can be seen in Table 1. Then, patients’ medical and laboratory data were collected. The study medical data included gender, the start date for taking warfarin, age (in years), indications, target INR range, and the duration of time in which the patient required to be treated. The patients’ laboratory data included the following information recorded at each visit: visit date, blood test date, INR result, complication type, complication incidence date, new dosage, next blood test date, and record date. During the project implementation (both the “Before” and “After” periods), patients were supposed to refer to former laboratories, and the quality of laboratory tests was also supposed to be constant.
Note: a Dosage based on multiples of 1/4 warfarin 5 mg tablet
b Multiple/extensive bruising: The area of bruising is about half the size of the palm or more than three in number
The evaluation of patients started from the “Before” period, and patients could move on to the “After” period, provided that at least four tests were performed on each patient. The minimum number of tests for transfer to the “After” period was four tests. Therefore, at the doctor's discretion, the number of tests in the “Before” period may have been 4 or more. Besides, the condition for the completion of the “After” period was to perform at least four tests. In the “Before” period, patients were treated as usual, and in the “After” period, the XrinA mobile application was used to tele control oral anticoagulation therapy. In this application, there was a possibility to set up two-way communication between the patient and the physician via the Internet and send a notification. In each period, the ratio of the number of INRs within the therapeutic range to the total number of tests in that period was calculated and expressed in percentage. Therefore, for each period, the percentage of INRs within the therapeutic range was calculated and the results of the two periods were compared. Then, in both the “Before” and “After” periods, the patients’ TTR was calculated and compared.
Research Ethics and Patient Consent
This study has the ethics code "IR.SBMU.RETECH.REC.1398.388", approved by Shahid Beheshti University of Medical Sciences.
Data Analysis
After patient selection, the date and results of INR test was transferred to Excel (columns A to M), computed, and the following results were obtained.
Column A: The solar date of INR test is written under “Date of Blood Test”.
Column B: The solar date is changed to Gregorian calendar under the same column using the following formula.
=IF(MOD(VALUE(LEFT(A4,4)),4)=0,(VALUE(LEFT(A4,4))-1)*365+(IF((VALUE(MID(A4,6,2))-1)<7,(VALUE(MID(A4,6,2))-1)*31,IF((VALUE(MID(A4,6,2))-1)>6,(VALUE(MID(A4,6,2))-1)*30+6)))+VALUE(RIGHT(A4,2))+INT((VALUE(LEFT(A4,4))-1)/4)+1,(VALUE(LEFT(A4,4))-1)*365+(IF((VALUE(MID(A4,6,2))-1)<7,(VALUE(MID(A4,6,2))-1)*31,IF((VALUE(MID(A4,6,2))-1)>6,(VALUE(MID(A4,6,2))-1)*30+6)))+VALUE(RIGHT(A4,2))+INT((VALUE(LEFT(A4,4))-1)/4))-466710
Column C: The result of the patient test is written in INR column.
Column D: The number of days after the previous test obtained through the following formula is written in column D: “Days Since Last Test”.
=IF (C4="","", B4-B3)
Column E: “INR Diff” column shows the INR ratio calculated based on the following formula:
=IF (C4="","", C4-C3)
Column F: “Previous INR Within Range?” column depicts if the previous INR was within the therapeutic range based on the following formula:
=IF (C4="","", G3)
Column G: “Current INR Within Range?” column depicts if the new INR is within the therapeutic range based on the following formula:
=IF (C4="","", IF (C4<$P$2,"Below", IF(C4>$P$3,"Above","In Range")))
Column H: “Scenario” column shows if the INR result is consistent base on the following formula:
=IF (F4=G4, F4,"Calculate")
Columns I, J, & K are used to get the INR difference ratio for calculating TTR.
Column “INR Diff Above Range”: This column shows how higher the present INR is from the patient’s therapeutic range based on the following formula:
=IF (C4="","", IF (H4="Above", ABS(E4), IF(F4="Above",ABS(C3-$P$3),IF(G4="Above",ABS(C4-$P$3),0))))
Column “INR Diff Within Range”: The present INR within the therapeutic range has been calculated based on the following formula:
=IF (C4="","", ABS(E4)-ABS(I4)-ABS(K4))
Column “INR Diff Bellow Range”: This column shows how lower the present INR is from the patient’s therapeutic range based on the following formula:
=IF (C4="","", IF (H4="Below", ABS(E4), IF (F4="Below", ABS(C3-$P$2), IF(G4="Below",ABS(C4-$P$2),0))))
Column L: The number of days in which the patients’ INR is within the therapeutic range has been calculated in “Days within Range since Last Test” based on the following formula:
=IF (D4="","", M4*D4)
Column M: The percentage of the days in which the patients’ INR is within the therapeutic range has been calculated in “Days within Range since Last Test” based on the following formula:
=IF (J4="","", IF (E4=0, IF (G4="In Range",1,0), J4/ABS(E4)))
According to data and the above formulae applied on Excel sheet, the results are written in Columns 5 and 6.
An Excel sheet of a patient is also attached (“Data Analysis.xls” file).
Twenty-one patients participated the “Before” period. Demographic information of patients is given in Table 2.
The factors contributing to patient warfarin uptake are listed in Table 3. The patients’ therapeutic range is presented in Table 4. The whole study period including “Before” and “After” periods ranged from 25/4/2021 to 22/5/2022 for 13 months. The “Before” period lasted from 25/4/2021 to 18/12/2021 for 8 months and the “After” period lasted from 15/11/2021 to 22/5/2022 for 6 months. The start date of the “After” period was not a fixed date for all patients, and each patient moved on to the “After” period as they met its entry criteria. Result regarding the number of tests and the percentage of INRs within the therapeutic range for each patient is presented in Table 5. Result regarding each patient’s TTR during the study is given in Table 6. The INRs within the therapeutic range and TTR of patients as well as their mean are given in Table 7. In the “Before” period, 0-60 % of patients’ INRs were in the therapeutic range and the percentage of TTR ranged from 0% to 70.9%. In the “After” period, 21.1-70 % of patients’ INRs were in the therapeutic range and the percentage of TTR ranged from 28.8% to 62.5%. In the “Before” period, the mean percentage of INRs within the therapeutic range and the mean percentage of TTR of patients were 31.5% and 34.3%, respectively. In the “After” period, the mean percentage of INRs within the therapeutic range and the mean percentage of TTR of patients were 41.4% and 50.1%. In the “After” period, the mean INRs within the therapeutic range and the mean TTR increased by 9.9% and 15.8%. Moreover, the minimum percentage of INR tests within the therapeutic range and TTR, which were zero in the “Before” period, increased to 21.1% and 28.8% (Figure 1).
Patient self-care covers a broad spectrum ranging from the lowest level to the highest level representing the full patient responsibility for the treatment, which means that at the lowest level of self-care, the patient participates to a lesser degree in taking care of himself/herself as compared to the health system, whereas at the highest level of self-care, the patient performs 100% self-care behavior and moves towards patient self-management (PSM). On the one hand, nowadays, patients with chronic diseases are actively involved in their own treatment and have greater cooperation with the treatment team in this sense [1]. On the other hand, studies have shown that the accuracy of dose prescription determined using the computer to achieve the INR target level is not less than that of experienced medical staff prescription [14,18,19]; therefore, the determination of dose rate using computer software has received increased interest from physicians and medical staff day after day [12,13]. Due to the set of these benefits, eHealth applications gain more popularity in this field and PSM is considered as the next step in the management of oral anticoagulation therapy [1]. The platform applied to self-management software includes both personal computers (PC) and mobile phones. It appears that PCs apply highly in medical centers, while mobile phones use more on patients. Because smartphones are almost always with the patient and possess the capability to set up two-way communications [20].
Therefore, self-management mobile applications pave the way for establishing continuous communication between the patient and the care team. The whole study period including Before and After periods ranged from 25/4/2021 to 22/5/2022 for 13 months. The Before period lasted from 25/4/2021 to 18/12/2021 for 8 months and the After period lasted from 15/11/2021 to 22/5/2022 for 6 months. The start date of the After period was not a fixed date for all patients, and each patient moved on to the After period as they met its entry criteria (Table 5). In 18 studies [4,5,7,12-14,21-34], the impact of patient self-management of oral anticoagulation therapy using eHealth applications on the percent of INRs within range and the percent of TTR were investigated. Out of 18 studies on therapeutic outcomes, 16 studies showed that patient self-management using eHealth apps improved therapeutic outcomes. Out of 5 studies [4,5,24,27,28] on INRs within the therapeutic range, 4 studies showed that patient self-management of oral anticoagulation therapy using eHealth applications increased the number of INRs within the therapeutic range. There was no negative impact on the number of INRs within the therapeutic range in any study. Regarding INR, the results of the present study indicated that the XrinA application increased the mean INRs within the therapeutic range by 9.78%; therefore, it is recommended to replace conventional methods with this method. However, it should be pointed out that according to other studies conducted in this field, 50% of major complications occurred when INR is within the therapeutic range [1].
In these circumstances, the use of this application by patients, facilitating access to the care team, can play an effective role in the prevention and reduction of injuries caused by complications and can be regarded as an appropriate alternative to conventional methods as well. Out of 17 studies (4, 5, 7, 12-14, 23, 25-34) on TTR, 14 studies revealed that patient self-management of oral anticoagulation therapy using eHealth applications led to an improvement of TTR values. In most of these studies, it was found that patient self-management using applications increased TTR by 0.7 to 15.4%, so that compared to the conventional methods, the range of TTR increased from 53.2-72.7 to 63.3-80.2 in new methods. The results from the present study indicated that in the new method focusing on the XrinA application, the percentage of TTR ranged from 0-70.9 % to 28.8-62.5% as compared to the conventional methods, which led to a 15.57% increase in the percentage of TTR, indicating that patient self-management of oral anticoagulation therapy using XrinA application based on this index can also be considered as an appropriate alternative to conventional methods. Concerning TTR results, other studies indicated that TTR can be considered as an appropriate alternative to previous models, provided that the percentage of TTR is not less than 60% in a management model and also there is at least 5 to 10% improvement in the percentage of TTR as well [7,33].
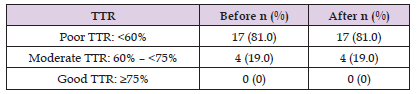
As presented in reference [34], TTR was divided into three ranges: weak, medium and good. In the current study, 81% of patients were in the weak range, although the proportion of patients with weak TTR did not alter in the “After” period (Table 8). However, it should be noted that TTR increased by an average of 15.8% compared to the conventional method, indicating that the patient self-management of oral anticoagulation therapy using the XrinA application can be regarded as an appropriate alternative to conventional methods. The INRs within the therapeutic range and TTR of patients as well as their mean are given in Table 8. During interviews with patients, it was found that the main cause of low adherence among patients in the Before period was the forgetting of drug use times and more specifically, not performing tests on time. Significantly, the use of XrinA application increased patients’ adherence. During the interview with the physician, it was revealed that another cause of being out of therapeutic range among patients was the culture of using herbal teas and subsequently the arbitrary use of herbal teas by the patients.
Table 9: Distribution of patients of the two OAT treatment groups (“Before” and “After” with the XrinA).
The study results indicated that the use of XrinA-based PSM improved therapeutic outcomes including the number of INRs within the therapeutic range and the percentage of TTR. However, there were some negative findings among some patients, revealing the necessity for proper application development and implementation and subsequent continuous evaluation. Setting up communication between the patient and the physician was among the other capabilities of the XrinA application. In order to develop mHealth applications with more features and capabilities, it is necessary to organize a team with a combination of different experts in the fields of information technology (IT) and medicine possessing the capability of developing appropriate applications with full knowledge. It is, therefore, recommended that mobile technology, due to its pervasiveness, be utilized in further studies to improve patient self-management of oral anticoagulation therapy.
The Authors declared that there is no conflict of interest.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
HM and MNH designed the research method. BSK analyzed and interpreted the patient data regarding the cardiovascular disease. LS edited the manuscript. All authors read and agreed upon the final manuscript.
There were no relationships with industry.
All data are incorporated into the article.
Mobile technology, due to its pervasiveness, can increase treatment adherence of the patients. Mobile application can be used as an assisting tool for Patient Self-Management (PSM). The study results indicated that the mobile health Patient Self-Management improved therapeutic outcomes including the number of INRs within the therapeutic range and the percentage of TTR.


