Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Tatiane Cristina Caldeira Ulzefer*
Received: March 01, 2024; Published: March 15, 2024
*Corresponding author: Tatiane Cristina Caldeira Ulzefer, Ave Coronel Lucas de Oliveira Number: 1955/ 201 Petropolis Porto Alegre, Rio Grande do Sul, Brazil
DOI: 10.26717/BJSTR.2024.55.008722
This study aimed to perform a clinical and tomographic evaluation of root coverage of Miller class I and II gingival recessions, treated with the tunnel technique associated with connective tissue graft (CTG) or leukocyte- platelet-rich fibrin (L-PRF) membrane. Eleven patients with bilateral recessions were selected, and a total of 59 sites, 29 in the control group (treated with connective tissue graft), and 30 in the test group (treated with leukocyte-platelet-rich fibrin membrane). Clinical evaluations of probing depth, clinical attachment level, amount of attached gingiva, and recession extension were performed at baseline and after three and six months of surgical procedure. Tomographic exams were performed to evaluate tissue thickness at baseline and after six months. The results showed that there was no difference in bleeding on probing and clinical attachment level between the groups. There was a significant reduction in the recession extension in both groups, without statistical difference between them after three months, and a higher reduction in the CTG Group after six months. The mean percentage of root coverage was 84.3% for the CTG group and 64.4% for the L-PRF group. The number of sites with complete root coverage was 55.2% for the CTG group and 33.3% for the L-PRF group. As regards the attached gingiva, the analysis of variance showed that L-PRF was associated with lower values after treatment (p = 0.005) when compared to CTG. The tomographic results of gingival thickness showed a statistically significant interaction between the treatments (p = 0.010). There was no statistically significant difference in gingival thickness in the L-PRF group between time points, while CTG promoted an increase in gingival thickness at six months. It could be concluded that CTG was more effective for root coverage, and to increase the tissue thickness and amount of attached gingiva than L-PRF after six months of evaluation.
Keywords: Conjunctive Tissue Graft; L-PRF; Root Recession; Tunneling
Abbreviations: L-PRF: Leukocyte- Platelet-Rich Fibrin; CTG: Connective Tissue Graft; PRP: Platelet-Rich Plasma; VAS: Visual Analogue Scale; RCF: Relative Centrifugal Force
Gingival recession is described as the apical migration of the gingival margin beyond the cementoenamel junction, exposing the tooth root surface [1]. It is a very prevalent problem affecting adults and children [2], and the most common etiological factors associated with gingival recessions are anatomical [3,4] pathological conditions [5], iatrogenic factors [6], and mechanical trauma [7]. Besides the aesthetic problems, gingival recessions can cause dentin hypersensitivity, difficulty in dental cleaning, carious and non-carious cervical lesions, and periodontal attachment loss [8]. The success of gingival recessions treatment is directly related to several factors that can influence the root coverage, such as the classification of gingival recession and the amount of proximal attachment loss, interventional factors, systemic factors, professional experience, and post-operative complications [9-11]. The connective tissue graft (CTG) technique described by Edel [12] is based on the fact that the connective tissue carries a genetic message that induces the epithelium to become keratinized. Studies have shown that the use of the coronal repositioned flap technique associated with CTG is the gold standard for root coverage treatment [1,13]. However, this technique has the main disadvantage of the need for a second surgical site, the palate is usually the donor area, increasing the patient discomfort. Because of these limitations, investigations regarding tissue substitutes, such as autogenous membranes from platelet concentrates, have been carried out in the last twenty years [14].
Since the removal of anticoagulants and the modification of centrifugation protocols, many essential aspects of tissue regeneration with platelet-rich plasma (PRP) have been described. Additionally, the introduction of leukocytes in platelet concentrates had a significant impact on the use of PRF on tissue regeneration and wound healing. PRF membranes are easy to prepare and manipulate, and when used for the treatment of gingival recessions, repairs the functional properties of the gingiva, and promotes the maintenance and integrity of the keratinized gingival tissue [15]. A split-mouth randomized clinical trial comparing the root coverage with connective graft or PRF showed statistically significant results in the degree of root coverage after six and 12 months for both approaches, with no significant difference between the two groups according to a systematic review by Miron & Choukroun [16]. However, when assessing the improvement in keratinized tissue, studies have shown that the CTG promoted a higher gain when compared to the PRF membranes [17,18]. The objective of this split-mouth randomized clinical trial is to compare the results obtained using the tunnel technique - a flap design that does not include the papillae, promoting superior aesthetic results - associated with CTG or PRF membranes in the root coverage, the gingival thickness (assessed by examination high-quality tomographic scan) and the keratinized tissue gain in Miller Class I and II gingival recessions.
Study Design and Patient Selection
The local Ethical Committee of São Leopoldo Mandic School of Dentistry (Campinas, Brazil) approved the study - number 92738518.0.0000.5374, and it was registered at Brazilian Clinical Trials Registry (REBEC) under the number RBR-4msz4x. Eleven patients between 28 to 62 years of age, ASA I (healthy patients, according to the physical status classification system of the American Society of Anesthesiologists - ASA), presenting Miller class I and II gingival recession (without interproximal attachment loss) were selected in a private clinic in Porto Alegre, Brazil. The sample size consisted of 59 teeth (29 sites treated with CTG and 30 sites treated with PRF).
The inclusion criteria were the presence of Miller's class I and II vestibular recessions in at least one tooth in each quadrant of the maxilla, from central incisor to the second premolar, in vital teeth, without bleeding on probing. Smokers, pregnant and lactating, and patients with systemic diseases (ASA II) were excluded from the study. It is a split-mouth single-blind randomized clinical trial, and the groups’ randomization was performed immediately before the surgical procedure. The recessions were divided in test group (treated with Tunnel technique + PRF; n = 30) and control group (treated with Tunnel technique + CTG; n = 29). On the postoperative visit, the patients completed a visual analogue scale (VAS) - a numerical scale ranging from zero to 10 - to describe the pain intensity observed in the recessions treated in the test or control groups. The CTG was removed from the palatal area on the same side that received the control treatment, favoring the accuracy of patients' responses on the VAS scale.
Clinical Examinations
The clinical examination was performed by two experienced and calibrated examiners (EJ and CFS), blinded to the treatments. The clinical parameters examined in each gingival recession were:
a) Visible plaque index: presence or absence of plaque deposits;
b) Clinical attachment level and probing depth at six sites per tooth;
Those measurements were performed at baseline, three and six months after the surgical procedure, using a 15 mm millimeter periodontal probe (North Carolina Hu-Friedy®).
c) Gingival thickness and amount of attached gingiva: measured in the central point of the buccal surface. The gingival thickness was measured using a Cone Bean tomography (Prexion 0.10 mm voxel tomograph) with soft tissue retraction. A linear measure of the soft tissue was performed 1mm above the bone crest in the most cervical point. The tomographic examinations were performed in the same radiological center and evaluated by a single examiner at the baseline (before the surgical procedures) and six months after surgery. The measurement is described in Figure 1.
PRF Preparation
The PRF was prepared according to the protocol described by Choukroun, et al. [14]. Immediately before the beginning of the surgical procedure, 10 ml of blood was collected by venipuncture into a sterile glass tube without anticoagulant. The blood was collected quickly, and the tubes were centrifuged for ten minutes using a relative centrifugal force (RCF) of 200 xg in the Montserrat Fibrinfuge 25® centrifuge (Zenith Lab Co, Changzhou Jiangsu, China). Due to the difference in density, the tube content was separated into three basic parts after centrifugation: red cells at the bottom, acellular plasma on the surface, and PRF in the middle of the tube. After the centrifugation cycle, autologous leukocyte and platelet-rich fibrin were produced on the tubes (Figure 2). They were removed from the tube, without the clot, with Dietrich tweezers, placed in a metal box and pressured to obtain a 1 mm thick membrane. The fibrin in the liquid phase (monomeric) was collected from the centrifuge tube with a sterile 3 ml transfer pipette (Shandong Weigao, Weihai, China) and used to glue the fibrin matrices. Three membranes were positioned on top of each other, agglutinated with fibrin in the liquid phase, and pressed in the metal box to form the final membrane. This process is described in Figure 3.
The Donor Area
For intra and extra oral disinfection, 0.12% and 2% chlorhexidine digluconate solutions were used, respectively. Local infiltrated anesthesia was performed using articaine 4% with epinephrine 1: 100,000 (Articaine®, DFL-Brasil). The CTG was harvested from the palate in the region between the first premolar and the second molar, depending on the needs of each patient. The CTG was removed with the bilaminar technique using a Swann Morton® blade [15]. The first horizontal incision was made 2 mm from the gingival margin, perpendicular to the palatal surface with 1.5 to 2 mm of depth. The second horizontal incision was parallel to the first one at a distance of 2 to 3 mm. The graft was removed with mesial and distal vertical incisions with a uniform thickness of 1.5 to 2 mm (Figure 4). The CTG was de-epithelialized (Figure 5) and kept in the saline solution until its use in the receptor area. The donor area was protected with PRF membranes and sutured with 5.0 nylon thread (Ethicon).
Receptor Area
The procedures were performed bilaterally on the maxilla, one side included in the control, and the other in the test group. Surgical procedures were performed by a single operator (TCU) with a magnifying loup (2.5x Surgitel Dent-All Innovation, Netherlands) and photophore. After local infiltrative anesthesia, root preparation was performed with scaling and planning of the gingival recession with Grayce curettes (Hu-Friedy®, RJ, Brazil), allowing the smear layer removal. This procedure produced a flat or negative root surface, relevant to the position of the CTG or PRF membrane under the flap with less tension. The area was irrigated with saline.
The subperiosteal incision was performed with Microblade Beaver (Swann Morton®, Sheffield, England) in the gingival margin of the treated and adjacent teeth, without releasing of the interdental papillae and favoring a full-thickness horizontal flap. Tunneling instruments (Hu-Friedy®, RJ, Brazil) were used to create a tunnel and enlarge the flap beyond the mucogingival junction. Apically, the flap was divided from the mucogingival line to ensures a coronal mobilization, tension removal, and passive coronary displacement of the flap.
The PRF membrane (three agglutinated membranes) or the CTG were inserted and adapted under the flap by the tunneling technique, covering the root recessions completely. Stabilizing sutures were performed on the mesial and distal portion of the graft with simple sutures using 5.0 nylon thread (Ethicon®, Johnson & Johnson). Suspensory sutures involving the contact point in the interproximal area were performed to advance the flap coronally and to obtain root coverage without tension. A 5.0 nylon thread was used (Ethicon®, Johnson & Johnson). Composite resin without acid conditioning was added on the contact points, before suture, to assist the mechanical stabilization of the suture (Figures 6 & 7).
Postoperative Care
Each patient was instructed to use ice pack externally in the operated area, in order to minimize edema and postoperative bleeding. Amoxicillin 875 mg (every 12 hours for seven days), ibuprofen 600 mg (every 12 hours for three days), paracetamol 750 mg (every six hours for as long as there was a pain) were prescribed. Mouthwash with 0.12% chlorhexidine digluconate (Periogard, Colgate-Palmolive), twice a day, was recommended for two weeks. The sutures were removed after 12 days, and, after that, the patients were instructed to perform oral hygiene using a soft toothbrush and reduced force. The healing procedure occurred naturally, and patients were reevaluated after one, three and six months of surgery.
Statistical Analysis
After the patients’ characterization, probing depth, and clinical attachment level were evaluated with descriptive analysis to determine the periodontal health of the subjects included in the research. The comparison of the efficacy of root coverage with CTG or PRF in the time points (initial, after three or six months), as well as the interaction between those factors, were analyzed with two-way repeated-measures analysis of variance (ANOVA). For multiple testing, Tukey's tests were used. Friedman tests were applied to compare the percentage of root coverage obtained with the treatments, and also to evaluate whether the treatment with PRF affected the postoperative pain assessed by the VAS scale. The statistical analysis was performed using the SPSS 23 (SPSS Inc., Chicago, IL, USA), with a significance level of 5%.
From the 11 patients included in the research, 59 superior teeth were treated: 3.4% central incisors; 10.2%, lateral incisors; 35.6%, canines; 33.9%, first pre-molar; and 16.9%, second premolars. In one patient, one tooth was treated with PRF and the contralateral tooth with CTG. In three patients, two teeth were treated with PRF and the two contralateral with CTG. Three patients had three teeth treated with PRF and the three contralateral with CTG. One patient had four teeth treated in each group. Three patients did not present the same number of teeth treated in each group, being two teeth treated with PRF and three with CTG or four teeth with PRF and three with CTG. The probing depth and clinical attachment level of the treated patients were described in Table 1. For the clinical parameter of the gingival recession, the two-way repeated-measures ANOVA demonstrated a statistically significant interaction between the treatment and the time point (p = 0.003). Both treatments presented a reduction in the gingival recession over time, with no statistically significant difference between three- and six-months measures. Although the test group presented a lower gingival recession at baseline (even with the randomization before the surgical procedure), there was no statistically significant difference between both treatments after three months. At six months, a lower gingival recession was achieved by the CTG group (Table 2 & Figure 8).
Table 1: Means and standard deviations of the probing depth and the clinical level of insertion (in millimeters) of teeth treated in the study.
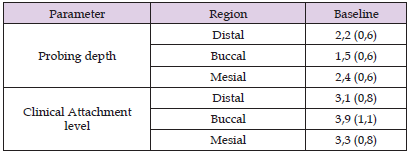
Table 2: Means and standard deviations of the gingival recession and the amount of attached gingiva, for each treatment and time point.
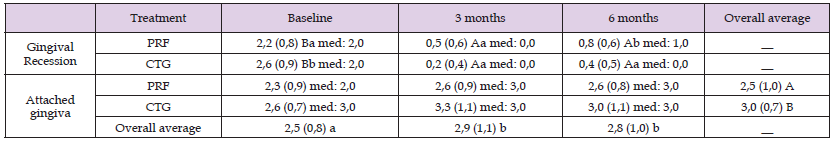
Means followed by different capital letters indicate a statistical difference between time points in each group. Means followed by different lower-case letters indicate a significant difference between treatments in each time point. General means followed by different capital letters indicate a statistically significant difference between treatments, regardless of time. General means followed by different lower-case letters indicate a statistically significant difference between times, regardless of treatment. Regarding the tomographic measures of gingival thickness, it was observed a significant interaction between the treatments and the time point (two-way repeated-measures ANOVA, p = 0.010). The PRF presented no statistically significant difference in gingival thickness over time, while the treatment with CTG promoted an increase in gingival thickness after six months. Although there was no difference between groups regarding gingival thickness at baseline, at six months, the CTG group presented higher tissue thickness (Table 3 & Figure 9).
There was no statistically significant difference (Friedman test, p = 0.194) in the percentage of root coverage between CTG (mean: 84.3%; standard deviation: 19.6%; median: 100%) and PRF (mean: 63.4%; standard deviation: 33.6%; median: 67%). Considering the frequency of the sites with complete root coverage or not, there is no statistically significant difference between groups (Fisher's exact test, p=0.119) (Table 4). Regarding postoperative pain (Figure 10), lower VAS scores were observed in the PRF group (Friedman test, p = 0.003). While the PRF sites experienced the average pain of one (minimum and maximum scores were zero and six respectively), the CTG sites presented the average of the pain of four (minimum and maximum scores of one and eight, respectively).
The high prevalence of gingival recessions in adults and the greater demand for root coverage treatment stimulate researchers to investigate alternatives to the CTG. This approach produces a second surgical site and, consequently, higher morbidity of the procedure. In the present study, PRF was evaluated as an alternative to CTG due to its characteristics of fibro-promotion and regeneration. Both methodologies used for the treatment of gingival recessions showed favorable results, considering that there was no statistical difference between treatments after three months. However, after six months, the sites treated with CTG obtained a 20% higher degree of root coverage compared to the sites covered with PRF. This difference was also observed on the percentage of complete root coverage, being 55.2% for the CTG group and 33.3% for the PRF group. A split-month, randomized, controlled clinical trial19, comparing the degree of root coverage using the coronal advanced flap (CAF) technique associated to CTG (n = 30) or PRF (n = 30), obtained 84% of root coverage in the CTG group and 77.12% for the PRF group after six months. The percentage of sites with complete coverage was 60% for the CTG and 50% for the PRF, with no statistical difference between groups. Similar results were found for Tunali et al. 18 in a split-mouth and randomized study, comparing the root coverage obtained with CAF associated with CTG (n = 22) or PRF (n = 22) after 12 months. The study achieved a degree of coverage of 77.36% and 76.63% for ETC and PRF, respectively, with no statistical difference between groups.
However, in the present study, the sites treated with CTG demonstrated superior results regarding the degree of root coverage and gingival thickness increasing. A possible explanation for this difference could be the surgical technique used in both groups. The study used the Tunnel technique in association with the CTG or PRF grafts. This technique has the advantage of not including the papillae in the flap, which, besides, to promote superior aesthetic results, also allows increasing the gingival thickness and the range of keratinized tissue. The tomographic results were used to evaluate the gingival thickness. When the PRF was used, there was no significant difference over time, while the CTG promoted an increase in gingival thickness after six months. (Öncü, et al. [19]) also described a statistical difference favorable to the CTG group regarding gingival thickness. Both studies used the bilaminar technique to remove the CTG, a methodology that recommends the removal of the CTG with the epithelium, and its de-epithelialization outside the mouth.
It favors the removal of a CTG with higher quality, with less adipose tissue and higher density, which promotes less contraction of the graft during the healing (Figure 11). Regarding the amount of attached gingiva, both treatments produced an increase of this parameter over time; however, favorable results were observed for CTG when the groups were compared. This result corroborates with previous systematic reviews [16,20,21]. (Tunali, et al. [18]) described no significant differences in the amount of gingiva attached after 12 months. The increase in attached gingiva in the CTG group can be explained by the fact that CTG maintains the genetic expression from the donor site, thus increasing keratinization and the gingival thickness in the receptor area [22]. Recent studies, with long-term follow-up (over five years), have shown that gingival margins with at least 2 mm of attached gingiva have better stability and can prevent gingival recession [23,24]. The lower keratinization observed in the PRF group is explained by the fact that autologous fibrin stimulates angiogenesis, inducing the formation of neovascularization and new tissues in the recipient area. For a successful procedure, the quality of the receptor area is crucial. Therefore, if a band of keratinized tissue is available, it will stimulate the formation of more keratinized tissue.
On the other hand, if only a non-adherent mucous tissue is presented, it will stimulate the formation of tissue with the same properties, with low quality and without attached gingiva [25]. Randomized clinical trials with more than twenty years of follow-up have emphasized the importance of tissue biotype for the gingival margin stability over time24. The techniques using the submerged CTG have obtained higher gain in the gingival thickness and in the band of keratinized tissue, modifying the tissue biotype. It is a characteristic of connective tissue to express its genetic condition on the epithelium [26,27]. This condition was also reported in the present study, once the gain in gingival thickness with CTG, measured through tomographic examinations, was significantly higher (0.4 mm) after six months. However, PRF promoted no significant gain in gingival thickness over time, achieving 0mm of thickness gain after six months. The present study used the protocol proposed by (Pinto, et al. [28]) to prepare the PRF membrane. According to it, three PRF membranes were positioned one over the other and agglutinated with autologous fibrin in the liquid phase before the insertion on the grafted area. The agglutination of PRF membranes is a relevant step of this technique once the quality and quantity of soft tissue obtained after root coverage with PRF membranes are directly related to the amount of fibrin matrix grafted [29].
It was demonstrated, through quantitative histomorphometric analysis, that fibro-promotion could be clinically predictable when using three to four PRF membranes per pair of teeth29. Scientific evidence indicates that PRF significantly improves the healing of hard and soft tissue [25,30]. Clinical procedures are benefited with the use of autologous fibrin28. In the present study, the side treated with root coverage associated with PRF, and so did not the need of a second donor site, presented more comfort and less pain in the postoperative period, with significantly lower values (median=0 and scores ranging from 0 to 6) when compared to the side receiving CTG and with a second donor area (median=4 and scores ranging from 1 to 8). It is important to observe that, in this study, the CTG was always removed from the same side that received the treatment with CTG [31]. The primary objective of periodontal plastic surgery in the treatment of gingival recessions is to improve aesthetic factors, to reduce the dentin hypersensitivity and the enlargement of keratinized tissue by covering the exposed root roots. The choice of the surgical technique is directly related to several factors such as the anatomy and location of the defect, the amount of adjacent attached gingiva, the number of teeth to be treated, donor area, professional's technical skills, patient's pain threshold.
Therefore, it is necessary to evaluate all these factors together, with the best available scientific evidence, for decision making. Recent scientific evidence indicates that the evolution of surgical instruments, less invasive surgical techniques, and the quality of sutures in the last two decades has positively converged to the periodontal plastic surgery results. Chambrone & Pini Prato13 and systematic reviews31 considered high scientific quality by the 2015 American Academy of Periodontology Workshop, support the evidence that the complete root coverage is directly related to the gingival recession depth (cervicoapical length) and to techniques that use CTG, considered the gold standard procedure due to its morphogenetic characteristics. Though, some factors limit its usage, such as the patient's refusal to undergo a second surgical site to obtain the CTG. Therefore, alternative strategies are necessary in cases where it is not possible to use it. PRF is one option due to its regenerative and fibro-promotion properties, and the ability to induce the formation of new fibroblasts when the receptor area presents a band of keratinized tissue.
However, in the present study, the increase in gingival thickness and the gain in keratinized tissue are restricted to the cases treated with CTG. Within the limitation of the present study, both grafts (PRF and CTG) used for root coverage of Miller class I and II gingival recessions showed favorable results. There was a significant reduction in the extent of the recession in both groups, with no statistically significant difference between them after three months; however, after six months, the results were significantly favorable to the CTG group. The average percentage of coverage was 84.3% for the CTG group and 63.4% for the PRF group. The percentage of sites with complete coverage was 55.2% for the CTG group and 33.3% for the PRF group. Regarding the attached gingiva, the PRF group is associated with significantly gain. The tomographic results of gingival thickness showed that there was no significant difference in gingival thickness for the PRF group, while CTG promoted a significant increase in gingival thickness after six months. Although several randomized clinical trials have been carried out to find tissue substitutes for the CTG in the treatment of gingival recessions, CTG remains the gold standard due to its biological and morphological characteristics. These properties are seen in the postoperative period, in which a thicker, denser, and keratinized gingival tissue is observed.
The use of the PRF membrane for the treatment of gingival recessions is limited. Its benefits are related to healing factors observed clinically in the donor area, accelerating revascularization and healing of the site, and reducing the patient's postoperative pain and discomfort during the wound repair. During the follow-up of gingival recessions treated with PRF, there was no change in tissue biotype, and most cases remained with thin tissue and reduced keratinized gingival, which is more susceptible to the gingival recession when in the presence of etiological factors.


