Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Mika Handa1, Tsuyoshi Takiuchi1,2*, Fumie Saji1, Tatsuya Miyake1 and Tadashi Kimura1
Received: March 04, 2024; Published: March 12, 2024
*Corresponding author: Tsuyoshi Takiuchi, Department of Clinical Genomics, Graduate School of Medicine, Osaka University, Department of Obstetrics and Gynecology, 2-2 Yamadaoka, Suita, Osaka 565-0871, Japan
DOI: 10.26717/BJSTR.2024.55.008714
Controlled Ovarian Stimulation (COS) is one of the crucial components of assisted reproductive technology. Progestin-Primed Ovarian Stimulation (PPOS) is a recently popularized COS method; however, the exact mechanism by which it suppresses ovulation remains unclear. kisspeptin, situated upstream of gonadotropinreleasing hormone (GnRH), promotes the secretion of follicle-stimulating hormone and luteinizing hormone, thereby inducing ovulation. In this study, we continuously monitored plasma kisspeptin levels in patients (n = 5) during PPOS. This study revealed no significant changes in plasma kisspeptin levels during PPOS, even immediately before oocyte retrieval. Additionally, we evaluated the expression changes of kisspeptin in the immortalized hypothalamic neuronal cell model mHypoA-50 following administration of the progestin used in PPOS. Progestin administration significantly suppressed kisspeptin gene expression in mHypoA-50 cells, which was increased with estrogen or GnRH stimulation. In conclusion, progestin may inhibit the increase in kisspeptin during PPOS, thereby suppressing ovulation.
Keywords: Kisspeptin; PPOS; Progestin; MHypoA-50
Abbreviations: COS: Controlled Ovarian Stimulation; PPOS: Progestin-Primed Ovarian Stimulation; GnRH: Gonadotropin-Releasing Hormone; ART: Assisted Reproductive Technology; FSH: Follicle-Stimulating Hormone; LH: Luteinizing Hormone; AVPV: Anteroventral Periventricular Nucleus; ARC: Arcuate Nucleus; CMA: Chlormadinone Acetate; SEM: Standard Error of the MEAN
The global prevalence of infertility is 8%–12%, with Assisted Reproductive Technology (ART) as an effective treatment method [1]. ART consists of three principal components, including oocyte retrieval, fertilization and embryo culture, and embryo transfer. Among these, the achievement of high-quality oocyte retrieval is of utmost importance [2]. The appropriate choice of controlled ovarian stimulation (COS) is crucial for high-quality oocyte collection. Recently, progestin-primed ovarian stimulation (PPOS) has become popular, although the exact mechanism by which it suppresses ovulation remains unclear [3]. Kisspeptin, a hypothalamic neuropeptide hormone encoded by the Kiss1 gene, acts through the hypothalamic kisspeptin 1 receptor (Kiss1R) to induce the release of endogenous gonadotropin-releasing hormone (GnRH), which in turn increases Follicle-Stimulating Hormone (FSH) and Luteinizing Hormone (LH) secretion from the pituitary gland, thereby inducing ovulation [4]. Kisspeptin plays a pivotal role in reproductive function by regulating the hypothalamic–pituitary–gonadal axis. One of the functions of progesterone (P4) in humans is to suppress GnRH and LH secretion, and thus ovulation, but the detailed mechanism of action remains unclear [5]. Estradiol (E2) in rodents acts on kisspeptin neurons in the Anteroventral Periventricular Nucleus (AVPV) and Arcuate Nucleus (ARC) regions, thereby adjusting GnRH secretion through positive and negative feedback, respectively [6].
Additionally, a rodent study revealed that P4 administration to the AVPV region suppressed ovulation [7], indicating that progestin may suppress ovulation in humans by inhibiting kisspeptin gene expression. In this study, we continuously monitored plasma kisspeptin levels during PPOS to investigate their changes. Further, we measured changes in kisspeptin expression with progestin administration in mHypoA-50 cells, which were derived from mouse Kiss-1-expressing neurons in the AVPV region.
Ethical Approval
The Ethical Review Board of Osaka University Hospital (No. 21113-2) approved this study. Samples were obtained from patients who signed written informed consent before inclusion and who underwent PPOS at the Reproduction Clinic Osaka, Osaka, Japan, from November 2021 to January 2022.
Serum and Plasma Hormone Profiles During PPOS
Table 1 shows the baseline characteristics of five patients during PPOS. Kisspeptin-54 was measured in plasma from days 2–5 of the menstrual cycle to the oocyte pick-up day using a commercial enzyme-linked immunosorbent assay kit (Peninsula Laboratories International, Inc.; San Carlos, CA) [8]. Blood samples were immediately transferred into a polypropylene tube that contains ethylenediaminetetraacetic acid on ice and then centrifuged for 15 min at 1,600 g at 4 ℃. Plasma layer samples were collected and stored at −80 °C. The concentrations of FSH, LH, E2, P4, and kisspeptin-54 are presented in Figure 1.
Table 1: Baseline characteristics of patients undergoing the PPOS protocol: serum and plasma hormone profiles.
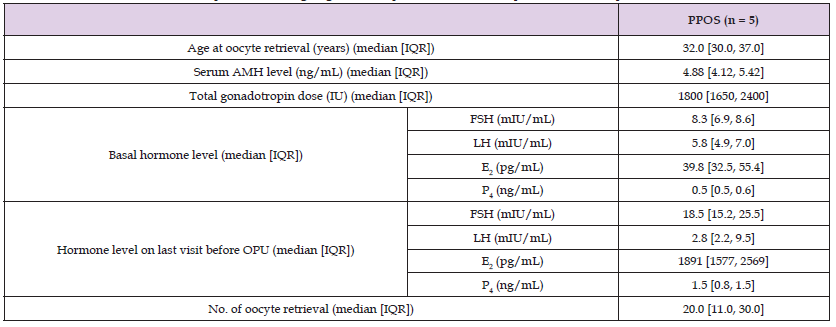
Note: AMH: anti-müllerian hormone; E2: estradiol; FSH: follicle-stimulating hormone; IQR: interquartile range; LH: luteinizing hormone; OPU: oocyte pick-up; P4: progesterone; PPOS: progestin-primed ovarian stimulation.
Materials
The following chemicals and reagents were obtained from the indicated sources: GIBCO fetal bovine serum (FBS) (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA); Dulbecco’s modified Eagle’s medium (DMEM) at 4.5 mg/mL of glucose (Invitrogen); phenol red-free DMEM (FUJIFILM Wako Chemicals, Osaka, Japan); charcoal-stripped FBS (Hyclone, Logan, UT); penicillin–streptomycin and water-soluble β-estradiol (Sigma-Aldrich Co., St. Louis, MO); and GnRH and Chlormadinone Acetate (CMA) (Fuji Pharmaceutical Co., Tokyo, Japan).
Cell Culture and Stimulation
CEDAR-LANE (Ontario, Canada) supplied mHypoA-50 cells. Cells were plated in 60-mm tissue culture dishes and incubated with high-glucose DMEM containing 10% heat-inactivated FBS and 1% penicillin–streptomycin at 37 ℃ under a humidified atmosphere of 5% CO2. The cell culture medium was changed to phenol red-free high-glucose DMEM with 10% charcoal-stripped FBS and 1% penicillin–streptomycin for a minimum of 24 h before the reagent and vehicle treatments in the mHypoA-50 cells. Each experiment used cells grown in culture plates to 80% confluence. Cells were incubated without (vehicle) or with the test reagents for the indicated concentrations and periods when stimulated with the test reagents. RNA preparation, reverse transcription (RT), and quantitative real-time polymerase chain reaction (PCR) A Nucleo Spin RNA Plus Kit (Takara Bio Inc., Shiga, Japan) was used to extract total RNA, and Super Script IV VILO Master Mix (Invitrogen) was used to synthesize cDNA from 2.5 μg of total RNA, following the manufacturer’s instructions. The following RT-PCR protocol was used for the initial identification of mPRα, mPRβ, and PgRMC1 mRNAs in mHypoA-50 cells. The forward primer for mPRα was 5ʹ-CAGAAGCCTCCGCAACCAGAAC-3ʹ, and the reverse primer was 5ʹ-GAGCCACAGCACTGAACGAGAG-3ʹ, whereas the forward primer for mPRβ was 5ʹ-TGACGACTGCCATCCTAGAGCG-3ʹ, and the reverse primer was 5ʹ-CAATGCCCCTGCCTCCACAAAG-3ʹ. The forward primer for PgRMC1 was 5ʹ-AGGGCAGGAACAGGTATGTG-3ʹ, and the reverse primer was 5ʹ-CCAAAGGAGTATTACCCAAGACC-3ʹ.
This primer sets generated products of 310, 305, and 205 bp for mPRα, mPRβ, and PgRMC1, respectively. Thermal cycling conditions were as follows: one cycle at 94 °C for 5 min; 35 cycles at 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 min; one cycle at 72 °C for 5 min. Amplified PCR products were run on a 2.0% agarose gel and investigated for appropriate size band production. Quantitative real-time PCR with Taqman™ Fast Advanced Master Mix (Applied Biosystems, Thermo Fisher Scientific, Waltham, MA, USA) was used to measure the mRNA expression of kisspeptin (Kiss1, Mm03058560_m1). Samples were run in triplicate using optical 96-well plates, and relative gene expression levels were evaluated using the 2−ΔΔCT method. Gene expression was normalized to histone 3a (Mm00517632_s1) mRNA levels, which were used as internal controls for the gene expression assay [9]. Each measurement is based on three biological replicates, and the values are presented as the means ± Standard Error of the Mean (SEM).
Statistical Analyses
One-way analysis of variance with the post hoc Tukey–Kramer test and GraphPad Prism version 8.0 were used for statistical analyses.
Plasma Kisspeptin Levels During PPOS
Table 1 shows the baseline characteristics of patients undergoing PPOS. The median age and anti-müllerian hormone level were 32.0 years (interquartile range [IQR]: 30.0–37.0) and 4.88 (ng/mL) (IQR: 4.12–5.42), respectively. While E2 serum levels continuously increased, no significant differences in plasma kisspeptin-54 and serum LH values were found during PPOS. No cases of ovulation were observed before oocyte retrieval.
Effect of CMA on Kiss-1 Gene Expression in mHypoA-50 Cells
We investigated the effect of CMA on Kiss-1 gene expression, which was increased with E2 and GnRH treatment, in mHypoA-50 cells. Expression of estrogen and GnRH receptors was confirmed in mHypoA-50 cells [9,10]. RT-PCR analysis revealed that the cells expressed mRNA for mPRα, mPRβ, and PgRMC1 (Figure 2a). E2 stimulation significantly increased Kiss-1 mRNA expression in mHypoA-50 cells by 1.47 ± 0.13-fold at 100 nM of E2 ([vehicle vs. E2], P < 0.05) and 1.06 ± 0.13- fold at 100 nM of E2 + 1.5 ng/mL of CMA ([E2 vs. E2 + CMA], P < 0.05) (Figure 2b). GnRH stimulation significantly increased Kiss-1 mRNA expression in mHypoA-50 cells by 1.41 ± 0.01-fold at 100 nM of GnRH ([vehicle vs. GnRH], P < 0.001) and 1.05 ± 0.07-fold at 100 nM of GnRH + 1.5 ng/mL of CMA ([GnRH vs. GnRH + CMA], P < 0.001) (Figure 2c).
COS enables the collection of a large number of oocytes in a single retrieval and improves cumulative pregnancy rates as the number of retrieved oocytes increases, thereby demonstrating their clinical efficacy [11]. However, ovulation suppressant administration is essential during COS to prevent breakthrough ovulation. The PPOS method uses progestin as an ovulation Inhibitors and is reported to be cost-effective and simple compared with other methods and yields reproductive outcomes, including ovulation suppression rates, comparable with those of other methods [3,12]. However, recent reports revealed a possible deterioration in the quality of embryos and a decrease in pregnancy rates [13,14]. Furthermore, slight increases in P4 levels before natural ovulation have potentially induced an LH surge, indicating that the exact mechanism by which P4/progestins suppress ovulation remains unclear [15]. The patients in our study were undergoing COS, and no cases of ovulation before oocyte retrieval were observed, confirming the ovulation-suppressing effect of progestin. Kisspeptin, a neuropeptide hormone that originates in the hypothalamus, is encoded by the Kiss1 gene and exerts its effect via Kiss1R located in the hypothalamus, thereby facilitating the release of endogenous GnRH [4]. Pubertal development was absent in families with Kiss1R mutations, thereby establishing an association between kisspeptin and reproduction [16]. Kisspeptin acts on various tissues, including the hypothalamus, ovaries, uterus, and placenta, thereby regulating their functions [6].
Kisspeptin in humans and many other species increases FSH and LH secretion from the pituitary gland, thereby triggering ovulation [4]. A single-blinded placebo-controlled physiological study [17] revealed that GnRH administration did not increase kisspeptin levels, indicating that kisspeptin operates upstream of GnRH. Two randomized, placebo-controlled, parallel-group, dose-finding trials (Phase I and Phase IIa) revealed that kisspeptin receptor agonist (MVT-602) administration induced an LH surge of similar amplitude to the physiological LH surge, indicating its use as a trigger for oocyte maturation [4]. Furthermore, the administered dose of MVT-602 induced a dose-dependent increase in LH levels [4]. Plasma kisspeptin concentrations increase before natural ovulation during the menstrual cycle and correlate with an increase in LH levels [18]. This study revealed that kisspeptin and LH concentrations did not increase before oocyte retrieval, indicating that LH surge suppression by progestin could be due to kisspeptin level reduction. The ovulation control mechanism via kisspeptin in rodents is different from humans, but rodents serve as an excellent model for investigating aspects that are difficult to analyze in humans. GnRH neurons in the hypothalamus do not express estrogen or P4 receptors in both humans and rodents [19]. There are two types of kisspeptin-producing neurons located in the hypothalamus: the Arcuate Nucleus (ARC) and the Anteroventral Periventricular Nucleus (AVPV) [19].
Estrogen suppresses Kiss1 gene expression in the former (negative feedback) and promotes it in the latter (positive feedback). Additionally, the former regulates pulsatile GnRH secretion (promoting follicular development), whereas the latter regulates surge-like GnRH secretion (inducing an LH surge and ovulation) [6]. In rats, kisspeptin neurons express estrogen and P4 receptors, which act on kisspeptin secretion from these neurons to regulate ovulation [20]. Studies have shown that P4 administration suppresses the LH surge, and RU486 injection (a P4 receptor antagonist) into the AVPV region increases LH secretion, indicating that suppression of the LH surge through P4 in rats involves AVPV neurons [20]. Estrogen administration in mice increases kisspeptin neurons in AVPV, whereas it decreases in ARC [21]. Furthermore, P4 administration suppressed the LH surge induced by estrogen administration, indicating the ovulation-suppressing effect of P4 [7]. However, the LH surge was not suppressed in groups administered both P4 and kisspeptin, indicating that P4’s ovulation-suppressing function may act upstream of kisspeptin, thereby controlling its secretion. mHypoA-50 cells, derived from mouse Kiss-1-expressing neurons in the AVPV region, have confirmed Kiss-1 and estrogen receptor expression, as well as GnRH/GnRH receptors [9,10]. Furthermore, we confirmed P4 receptor expression in mHypoA-50 cells and revealed that progestin (CMA) suppresses kisspeptin gene expression induced by E2 or GnRH.
The mechanisms of ovulation control differ between rodents and humans, but progestin in humans may suppress kisspeptin secretion through upstream mechanisms, thereby inhibiting LH surge occurrence. Plasma kisspeptin levels in nonpregnant adult women vary according to previous reports. Previous studies have reported plasma kisspeptin concentrations to be 1.65 ± 0.1 ng/mL [mean ± SEM], consistent with our findings [18]. Other studies have documented both lower [22] and higher concentrations [23] than those observed in our examination. The half-life of kisspeptin is short [4,24], and it is easily degraded, making the sample collection and preservation methods crucial. Studies retrospectively investigated the relationship between plasma kisspeptin levels and miscarriage [8,25] have also been published, proving the feasibility of measuring serum kisspeptin concentrations. Our study used the same assay system. Furthermore, our research did not establish a control group without the use of ovulation suppressants in COS, thereby not directly proving the suppression of plasma kisspeptin by progestin. However, conducting COS without ovulation suppressants is ethically untenable. An increase in estrogen levels can induce an LH surge, while plasma kisspeptin concentrations increase before ovulation even in natural menstrual cycles with lower estrogen levels [18]. Therefore, kisspeptin levels are expected to increase in this COS if progestin had not been used. Further research with an increased sample size is essential to confirm our study results. In summary, our human plasma analysis during PPOS and the additional experiments with mHypoA-50 cells indicate that PPOS with progestin significantly suppressed the LH surge rate, which may be due to the suppression of Kiss-1 gene expression in the hypothalamus.


