Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Vibhor Chaswal and Arshad Khan*
Received: February 15, 2024; Published: March 07, 2024
*Corresponding author: Arshad Khan, Department of Chemistry, Eberly College of Science, Pennsylvania State University, DuBois, PA 15801, USA
DOI: 10.26717/BJSTR.2024.55.008705
Some years ago, Lecker & Khan (Biotechnology Progress, 1996, 1998) examined the effect of heat on α-amylase by UV-VIS spectroscopy and put forward a two-step inactivation mechanism for this enzyme. In the first step Ca2+ ion dissociates reversibly from the active enzyme followed by irreversible inactivation of the apoenzyme (enzyme without the calcium attached). The purpose of this FTIR (Fourier-transform infrared spectroscopy) study is to examine what happens at the molecular level when the protein is heated from 20 to 90 °C. In addition to the known peak at 1636 cm-1 for C=O groups in proteins, two new peaks develop at around 1033 and 3000 cm-1 suggesting stretch frequencies for C-O (single bond) and O-H bonds respectively indicating the formation of C-O-H bonds in the protein. We propose a mechanism by which protein undergoes structural changes at high temperatures and forms new C-O-H bonds (Graphical Abstract).
Keywords: Amylase at High Temperature; Structural Changes in Amylase; Development of New IR Peaks at 1033 and 3000 cm-1
α-Amylase is an enzyme that hydrolyses α-linked polysaccharides, such as starch and glycogen, yielding shorter chain sugar molecules like glucose, dextrins, and maltose [1]. This protein is present in saliva and pancreatic secretions and is responsible for the digestion of starch. Because of its medical and industrial applications, researchers around the world have studied this enzyme extensively. It contains at least 1 mol of Ca2+ ion [2-5] per mole of protein. The strength of the binding of calcium ions to the protein varies significantly from one enzyme source to another. Irrespective of the source, α-amylase causes a rapid fragmentation of starch molecules into sugars [6-8] and undergoes inactivation reaction initiated by an initial dissociation of Ca2+ ions followed by denaturation by heat [9-11]. The theory of inactivation of α-amylase was developed by our group some 30 years ago and we confirmed the validity of its predictions by quite a few experiments in aqueous [9,10] as well as non-aqueous solutions [11]. The earlier UV-Vis studies primarily focused on irreversible inactivation step of the protein and examined the effects of heat, solvent type, and salt concentrations on the inactivation [9-11] reaction. In each case the two-step inactivation model is shown to be valid suggesting that this metallo-enzyme inactivates by first dissociation of the metal ion followed by denaturation of the apoenzyme (enzyme without the metal ion attached). Although the above UV-Vis studies allowed us to establish the two-step inactivation mechanism of this protein, new studies were required to understand what happens to the enzyme at the molecular level when the temperature is increased. With these questions in mind, we decided to carry out FTIR (Fourier Transform Infra-red) studies on this protein at different temperatures. Before we discuss these FTIR results, we plan to discuss the theory of inactivation that we developed earlier [9-11] followed by experimental section and the sections on results with discussions.
The first reversible stage (eq 1) involves a forward reaction that forms an inactive apoenzyme, E2-, from the active enzyme, CaE, with a rate constant of k1 and a reverse reactivation reaction involving the combination of E2- with calcium ions with a rate constant of k-1. The second irreversible stage (eq 2) of reaction forms a denatured form of the enzyme, EI2-, from E2- with a rate constant of k2. The reversibly inactivated form, E2-, can be quickly transformed into the active form, CaE, by adding calcium ions. On the other hand, the EI2- is the denatured form of the enzyme that cannot be reactivated by adding calcium ions.
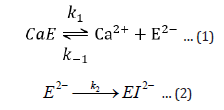
Based on inactivation steps 1 and 2, the following equations are derived:
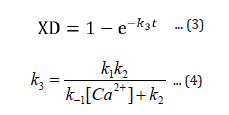
The expression XD (eqn 3) gives the fraction of enzyme inactivated at a time, t, after the inactivation process begins, and k3, given by equation 4, is a function of calcium ion concentration and temperature. From the expression 3 one can readily obtain the value of percent active enzyme as follows:
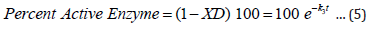
A more detailed derivation of equation 3 (and hence, eqn. 5) as well as various test results can be found in references [9,10].
Solution Preparation
The pure amylase solution of 6.540 x 103 mg/L concentration was prepared by dissolving solid (50,000 BAU/g) in pure water at 20°C. The other solutions were prepared at 50 and 90° C. The amylase from bacillus species was purchased from Sigma Aldrich Chemical Co.
Heating Details
Heating was done on a lab hot plate. The amylase solutions at 50 and 90 °C were prepared by heating solutions to the desired temperatures followed by their cooling to 20 °C before taking the spectral readings. Interestingly, in the process of achieving the highest possible temperature we noticed that the protein solution started boiling at around 90 °C. This is significantly lower temperature than the boiling temperature of water and may be due to structural changes in protein resulting in increased water vapor pressure and hence, lowering of its boiling temperature.
FTIR Spectra of Solutions
Initially spectrum of air background was taken followed by those of pure distilled water and amylase solutions at 20, 50 and 90 °C (cooled to 20 °C) by using Thermo Scientific Nicolet iS5 FT-IR spectrometer.
Figure 1 represents spectra of α-amylase solutions at 20 (black spectrum), 50 (red) and 90 °C (green). We labeled 5 major peaks/ peak bunches with numbers 1-5. Most of the spectral features like those around 2, 3 and 5 remain unchanged when the temperature is increased from 20 to 90 °C. However, peaks around 1 (around 1033 cm-1) and 4 (around 3000 cm-1) show significant changes with temperature, especially at 90 °C (green spectrum) with a sharp rise in peak intensity at 1033 cm-1. We assign peaks around 3 (around 2350 cm-1) and peak bunches around 2 and 5 primarily to water molecules present in the protein solution. We confirm this assignment by examining the FTIR spectrum of water published at NIST site (National Institute of Science and Technology) and is presented in Figure 2. In Figure 2 the water spectrum is in transmittance units. The corresponding absorbance spectrum can be obtained by simple inversion of the transmittance spectrum. As can be seen, in the water spectrum, there are two major peak bunches from 1500-1800 cm-1 (this is also present around 2 of our Figure 1 spectrum), 3500-4000 cm-1 (also present around 5 of our spectrum), and a small peak around 2300 cm-1 (present around 3). As mentioned above, all these spectral features are present in our protein solution (Figure 1) and suggest features due to water. After subtracting the spectrum due to water, we obtain distinct peaks at 1033 cm-1 and 1636 cm-1 and present them in Figures 3 & 4 respectively. In both Figures 3 & 4 temperature effects on peak intensities are shown. In Figure 3, one can notice that there is no 1033 cm-1 peak at 20 °C (black spectrum). However, at 50 °C (red spectrum) the peak is quite visible that rises sharply in intensity at 90 °C (green spectrum) giving a peak height of around 4 times that of the 50 °C peak.
Figure 4 shows a distinct peak at 1636 cm-1 at every temperature from 20-90 °C and is due to carbonyl groups (C=O) in proteins. This assignment is consistent with those reported in references [12-14]. Indeed, helical and pleated sheet structures of protein as presented in Figure 5 show carbonyl groups that absorb at 1636 cm-1. In contrary to peak intensity at 1033 cm-1, the 1636 cm-1 peak intensity shows almost linearly increasing intensity with temperature. This gradual rise in peak intensity at 1636 cm-1 with temperature may be due to the heat effect that gradually exposes a larger number of carbonyl groups to IR radiation. We assign 1033 cm-1 peak to C-O single bonds and peak around 3000 cm-1 to O-H bonds. So, the peaks around 1033 and 3000 cm-1 suggest formation of new C-O-H bonds. A possible mechanism for the formation of C-O-H bonds in protein at a high temperature is presented in Figure 6.
α-Amylase inactivates in two stages; a reversible stage of the dissociation of metal ion followed by irreversible inactivation of apoenzyme resulting in the denaturation of the protein. The present FTIR study focusses on the denaturation stage and indicates structural changes that happen at higher temperatures. As the temperature is increased from 20 to 90 °C two new absorbance peaks at 1033 (C-O stretch) and 3000 cm-1 develop that we assign to C-O (single bond) and O-H bonds. These stretch frequencies can be explained by considering the formation of new C-O-H bonds within the protein at higher temperatures. As expected, these structural changes were irreversible as cooling to 20 °C did not change the spectral features.


