Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Maksimovich NYe, Bon EI*, Ostrovskaya OB and Kurochkina ED
Received: February 20, 2024; Published: March 01, 2024
*Corresponding author: Elizaveta IBon, Candidate of biological science, Assistant professor of pathophysiology department named D. A. Maslakov, Grodno State Medical University; Grodno State Medical University, 80 Gorky St,230009, Grodno, Belarus
DOI: 10.26717/BJSTR.2024.55.008690
Electron microscopy is the leading method for studying the ultrastructure of cells. The main method of preparing tissue for research is contrast. Tissue sections, even fixed in solutions of O3O4 or KMnO4, i.e. in solutions that simultaneously serve as both fixative and contrasting agent, are usually insufficiently contrasted. Greater contrast can be achieved by selectively increasing the density of the structures. High molecular weight contrast agents, preferably heavy metals and their compounds, are suitable for this purpose. However, organic compounds are also suitable if they are bound by the tissue in sufficiently large amounts per unit volume (e.g. the azo dye hexazonium- pararararosaniline). When contrasting biological material, the increase in electron density is determined by the total number of atoms or molecules of the contrast agent captured by the tissue.
Keywords: Methodological Approaches; Contrasting; Electron Microscopy
Tissue sections, even fixed in solutions of O3O4 or KMnO4, i.e. in solutions that simultaneously serve as both fixative and contrasting agent, are usually insufficiently contrasted. Greater contrast can be achieved by selectively increasing the density of the structures. High molecular weight contrast agents, preferably heavy metals and their compounds, are suitable for this purpose. However, organic compounds are also suitable if they are bound by the tissue in sufficiently large amounts per unit volume (e.g. the azo dye hexazonium-pararararosaniline). When contrasting biological material, the increase in electron density is determined by the total number of atoms or molecules of the contrast agent captured by the tissue. Thus, the final electron density depends on the mass of molecules (atoms) of the contrasting substance, on the number of binding groups in the tissue and on the number of molecules (atoms) of the substance captured by the tissue. It follows, in particular, that the contrast intensity is inversely dependent on the thickness of the slice [1,2].In the process of contrasting there is often an extraction of various tissue components, which may be accompanied by both contrast enhancement and, on the contrary, its partial decrease [3].Elements used for contrast include: lead, uranium, tungsten, osmium, manganese, as well as iodine, iron, mercury, barium, vanadium, chromium, gold, silver, nickel, lanthanum, ruthenium, platinum, thallium, indium, bismuth, strontium, and thorium. In this manual, we will focus on only a few commonly used contrast agents that can be used in conjunction with numerous histochemical procedures. In addition, methods for selective contrasting of specific structures also deserve mention. Heavy metal salts with selective contrasting action will be discussed below in the relevant section.
The remarkable qualities of lead salts as a contrasting agent have long been recognized. At present, according to Watson, lead salts are used mainly in alkaline solutions. The use of lead salts as a contrast agent is complicated by the formation of insoluble lead carbonate and other undesirable precipitates [4]. To overcome this disadvantage, a number of modifications of the method have been proposed, among which the Reynolds and Karnovsky modifications are the most popular. Although both methods give a fairly stable solution of lead salt, attention should still be paid to the purity of the reagents used: they should be fresh and free of CO2 admixture (NaOH granules without film, fresh distilled water). The contrasting effect depends on the choice of the preceding fixation. The different methods of contrasting in alkaline medium seem to be based on the same mechanism, as they all give similar results. According to Reynolds, divalent lead salts in an alkaline medium (pH=12) form so called "basic" salts:
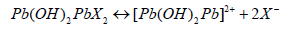
The connection of divalent cations (with two lead atoms) with phosphate, carboxyl and sulfhydryl groups, as well as with negative charges of osmium oxide, is apparently carried out through ionic bonds or through hydrogen bridges, as is probably the case with glycogen [5]. Lead is poisonous! Inhalation of its vapours and ingestion through the mouth and skin should be avoided!
The uranyl cation binds to phosphate and carboxyl groups. Uranil acetate UO2(CH3COO)2 (uranyl nitrate or double salts are used less frequently) is used in aqueous or alkaline solutions after fixation, during dehydration or when contrasting sections. In 20 min uranyl cation from aqueous solution is able to penetrate into the tissue filled in the eponge to a depth greater than the thickness of the ultrathin slice. In solutions of lead salts the diffusion rate is even higher [2,6]. Zobel and Veer found that at pH=3.5, 7 times more uranyl acetate binds to DNA than to bovine serum albumin [7]. At pH=5, the ability of both of these substances to bind to uranyl acetate increases and the difference in contrastability decreases. The effect of differential contrasting can also be observed at different concentrations of the contrasting agent. Thus, DNA is contrasted already at uranyl acetate concentration equal to 10-5M, while myosin is contrasted only at concentrations above 10-3M. The contrastability of lyophilised tissue with worse structure preservation was much better.
Significant contrast enhancement can be achieved by double contrasting ultrathin sections first with uranyl acetate and then with lead according to Reynolds or Karnovsky. Double contrasting using solutions of lead salts on slices or simultaneously on tissue pieces also gives good results [8]. In tissues fixed in aldehyde, contrastability with uranyl salts and/or lead hydroxide can be improved by treating the material with alcoholic solution of dinitrofluorobenzene at pH=8. The contrastability of the fabric with potassium permanganate or phosphorus-tungstic acid does not change.
General contrasting by phosphorus-tungstic acid H3P(W3O10)4 (mol. weight 2880) is carried out due to its interaction with proteins, the electron density of which can almost double. Proteolysis impairs the contrastability of phosphorus-tungstic acid. Like uranyl acetate, phosphorus-tungstic acid is used in aqueous solutions as well as in alcohol or acetone solutions to contrast both tissue pieces and slices. Because phosphoric tungstic acid strongly extracts some tissue components, it is best used mainly for contrasting slices, and its effect on tissue should not be prolonged [9]. The contrast results depend on the pretreatment of the tissue and on the pH of the phosphorus-tungstic acid solution. After fixation in osmium tetra oxide, the affinity of tissue to phosphorus-tungstic acid is partially masked, but it can be brought back to the initial level by exposure to oxidants (2% H202, 30-60 min) or longer treatment in phosphorus-tungstic acid. Material fixed in KMnO4 is not suitable at all [10]. Phosphorus-molybdenum acid is also suitable for contrasting, but it is weaker in this respect than phosphorus-tungstic acid.
With KMnO4 it is possible to obtain a strong contrast in slices, which can be further enhanced by subsequent contrasting with lead. Contrasting in slices is easier, but it involves, as in the case of fixation in KMnO4, a marked extraction of substances from the tissue and may lead to the disappearance of ribosomes and rupture of membranes [1]. In material treated with an aqueous solution of strontium permanganate, contrast is observed only in cut structures into which the contrasting agent can diffuse from the cut surface. Apparently, even a thin layer of resin makes it difficult to contrast with permanganate.
Zeligman et al. described a new imaging principle with general application for both light and electron microscopy. According to this method, metallophilic reagents are introduced into the tissue, the localisation of which is then revealed on electron micrographs due to the subsequent capture of metal ions during the contrasting process. Reagents with two or more functional groups are suitable for simple contrasting. They react primarily with osmium, which is bound in the process of fixation, but, in addition, retain the ability to form additional bonds with the same or with another metal. The most active ligands were thiocarbohydrazide H2NNHCSNHNH2 and carbohydrazide. The first of them is recommended for general contrasting by the OSO4- thio carbohydrazide - OSO4 method (OTO-method) [4]. In such contrasting, a pigment, the so-called "osmium black", is formed, which is considered as a copolymer. In model experiments after fixation in OSO4, lipids are most intensively blackened; proteins and nucleic acids react much weaker, and starch does not react at all. Unlike other methods, the intensity of contrasting by the OTO-method corresponds to the true osmiophilia of the tissue. Membranes are sharply delineated and appear thinner, apparently due to the strong contrast of the inner lipid layer. If contrast is found to be insufficient, the procedure can be repeated. This procedure, carried out according to the scheme: metal-thio carbohydrazide- OSO4 or metal-thiocarbohydrazide-metal (MTO- or MTM-reaction), can also be used to detect other cations. Pd, Os, U, Pb, Hg, Fe, Cr, Cu, Ca, Zn, Sn give positive reactions [2]. Magnesium and aluminium salts give a negative reaction. Similarly, the metals in the histochemical reaction product can be used to enhance contrast.
After conventional fixation in OSO4, intensive impregnation of individual structures can be achieved by longer treatment in an unbuffered solution of osmium tetraoxide at elevated temperature. With the help of such osmation it was possible to demonstrate the directionality of chemical synthesis processes in the Golgi apparatus. In cells of the cortical layer of the adrenal gland (rat), substances in the endoplasmic network and in mitochondria are contrasted. In addition, the outer segments of retinal bacilli of amphibians and gastropods and the outer segments of photosensitive cells of the Rana pineal organ are osmised, apparently due to photopigment. Fat droplets are not contrasted [11]. Osmation of material fixed in glutaraldehyde or in glutaraldehyde with acrolein gives the same results as fixation in 0S04. Blocking the aldehydes with dimedone has no effect on the reaction results. Pretreatment with mercuric (II) chloride releases additional reactive groups [12]. The chemical affinities of the substances that can undergo osmation have not yet been identified. However, some lipids are thought to be involved in the reaction, although cholesterol and neutral fats and possibly aldehydes do not seem to play a role. Perhaps the reason should be sought in the peculiarities of solubility of lamellae of the outer segments of the rod (but not cones) of the frog retina, as well as in the data obtained with the 0S04-zinc iodide reaction on sensitive cells of the rat retina [8].
Incubation of fresh tissue in medium with 0S04 and ZnI2 gives intensive contrasting of different cell components: Golgi apparatus, lysosomes, contents of perinuclear space, granular endoplasmic network, mitochondrial matrix, cytoplasm in nerve and glial cells, synaptic vesicles, granules in cells of islets of Langerhans, keratohyalin granules in epidermis, as well as granules in eosinophilic leukocytes, sensitive cells of retina, cells of the brain layer of adrenal gland, etc [13]. A positive reaction is only observed in certain cells, but even in these cells identical structures can react differently. Little is yet known about the substances involved in this reaction. On the grounds that a negative reaction was obtained after lipid extraction, the lipids or lipoproteins which appear to acquire the ability to react with 0S04 as a result of exposure to zinc iodide are thought to be responsible for the development of the colouring. At one time it was assumed that this staining was selective for cholinergic nerve endings, but later it turned out that synaptic vesicles in adrenergic fibres can also stain. On the basis of the available data, it can be assumed that this method is based on the involvement in the reaction of a certain grouping in lipids or in lipid-containing compounds. Any histochemical conclusions based on this reaction are not yet possible [6,14]. However, for contrasting certain structures on a purely empirical basis, the 0S04-zinc iodide reaction may prove useful.The method of contrasting with bismuth-iodine complex is similar to this method.
Numerous attempts have been made to modify the various silvering methods used in light microscopy: after all, the presence of metallic silver deposits at the reaction site is a good prerequisite for electron microscopic studies. Quite good impregnation is achieved by treating ultrathin sections of tissue fixed in 0S04 with a solution of methenamine-silver or borax-AgNO3. In such treatment, cell nuclei, mitochondria, cell membranes, as well as mucus, glycogen, collagen fibres and other structures are exposed to silvering. When treated with the same silver solutions of tissues fixed in acrolein or glutaraldehyde, only chromatin and ribosomes, as well as granules of eosinophilic leukocytes (especially their inner part) and granules of enterochromaffin cells are intensively impregnated; reticular and collagen fibres are impregnated somewhat weaker; mitochondria and nuclei in this case are usually free of silver deposits [15-18]. After fixation in formalin the impregnation is much weaker; a marked contrast is observed only in structures having a pronounced reducing power. There is a method of selective detection of nuclei. When treated with silver nitrate solution in vivo, intensive silver deposition is observed in connective tissue, although not in the cells themselves.The available data indicate that the results depend significantly on the pretreatment of the tissue and the choice of silvering method. Therefore, the specificity of the silver impregnation method is low [9]. The reduction of silver to free metal is mainly due to aldehydes, sulfhydryl groups, histones (i.e. arginine and histidine-rich proteins), and unsaturated lipids that bind lower oxides of osmium. Interpretation of the results is often difficult.
Nevertheless, if certain conditions are met, silver impregnation often allows important histochemical conclusions to be drawn.For general contrasting purposes, silvering is unlikely to be suitable due to the fact that silver deposits precipitate as granules. However, for the detection of morphological details or specific cells and to establish their spatial location, this method may be useful. Examples include the silvering of connective tissue fibres, mitochondria and various structures in nervous tissue by the Nauta or Golgi method, and the silvering of the cell wall in endothelium. These are all entirely empirical methods, and little is known for certain about their mechanisms. Histochemical interpretation of these data is therefore very difficult. Valverde believes that the Golgi method is based on the formation of a lipoprotein-chromium-silver compound by the catalytic action of 0S04. When the tissue is simultaneously exposed to osmium tetraoxide and some other oxidising agent (e.g. potassium bichromate), the mechanism of action of the former is reversed; the reduced osmium is immediately reoxidised to 0S04 [12]. In addition, those numerous compounds that 0S04 under normal conditions oxidises, in a mixture of osmium tetraoxide with the second oxidant do not react. In Golgi staining 0S04 reacts with ethylene groups of lipids. Under the action of bichromate, however, these bonds are broken again. Then bichromate oxidises also the released two OH-groups of ketone bodies, whose compounds with chromium react with silver nitrate, which leads to blackening of the tissue.
The Journal of the Acoustical Society of America 115(3).


