Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Gian Maria Pacifici*
Received: February 12, 2024; Published: February 28, 2024
*Corresponding author: Gian Maria Pacifici, Professor of Pharmacology, Via Sant’Andrea 32, 56126 Pisa, Italy
DOI: 10.26717/BJSTR.2024.55.008684
Meropenem is a carbapenem antibiotic and has a broader spectrum of activity than most β-lactam antibiotics, does not require co-administration with cilastatin because is not sensitive to renal dipeptidase, is available for intravenous infusion, is renally cleared, and the dose of meropenem is 1 or 2 grams thrice-daily. The efficacy and safely of meropenem, the prophylaxis with meropenem, the treatment of bacterial infections with meropenem, and the trials conducted with meropenem have been reviewed. The pharmacokinetics of meropenem have been studied in patients with serve sepsis or with septic shock and in healthy subjects and the mean elimination half-life of meropenem is 3.30 and 0.61 hours (P-value < 0.05) in patients and in healthy subjects, respectively. The concentration of meropenem in human tissues has been reviewed; meropenem poorly penetrates into the brain whereas it penetrates into lung, bronchial mucosa, and pleural tissue in concentration higher the MIC of most respiratory pathogens. The penetration of meropenem into the human cerebrospinal fluid has been reviewed and meropenem poorly penetrates into the cerebrospinal fluid. The median elimination half-life of meropenem is 0.63 and 13.86 hours in the serum and in the cerebrospinal fluid, respectively, and the median absorption half-life of meropenem is 23.10 hours in cerebrospinal fluid. Meropenem successfully treats bacterial meningitis and significantly reduces the serum concentration of valproic acid. The aim of this study is to review meropenem efficacy and safely, prophylaxis, treatment, trials, pharmacokinetics, tissue and cerebrospinal fluid concentration, treatment of bacterial meningitis, and interaction with valproic acid.
Keywords: Bacterial-Meningitis; CSF; Drug-Interaction; Efficacy-Safely; Pharmacokinetics; Prophylaxis; Tissue-Concentration; Treatment; Trials
Meropenem is a carbapenem antibiotic. Carbapenems are β-lactams that contain a fused β-lactam ring and a five-member ring system that differs from the penicillins because it is unsaturated and contains a carbon atom instead of the sulphur atom. This class of antibiotics has a broader spectrum of activity than most other β-lactam antibiotics largely due to their greater resistance to β-lactamase-mediated hydrolysis. Meropenem is a derivative of thienamycin. It does not require co-administration with cilastatin because is not sensitive to renal dipeptidase. It may be co-formulated with the β-lactamase inhibitor vaborbactam [1].
The spectrum of activity of meropenem is against gram-positive and gram-negative organisms [1].
Meropenem is available for intravenous administration and is renally cleared with an elimination half-life of about 1 hour. Although typically infused over 30 min, extending the infusion over 3 hours can increase the time that meropenem concentrations spend above the organism’s MIC and allow for treatment of low-level resistant pathogens. Meropenem and other carbapenems significantly lower serum concentrations of the antiepileptic agent valproic acid and should not be co-administered with this drug [1].
Meropenem is typically employed for hospital-onset infections of the respiratory, gastrointestinal, and urinary-tract when cephalosporins- or penicillin-resistant organisms are suspected (dosed 1 to 2 grams thrice-daily in patients with normal renal function). Meropenem is reserved for multidrug-resistant gram-negative pathogens (2 grams of meropenem administered thrice-daily in patients with normal renal function) [1].
Meropenem molecular structure (molecular weight = 383.46 grams/mole).
The literature search was performed electronically using PubMed database as search engine and the following key words were used: “meropenem efficacy, safely”, “meropenem prophylaxis”, “meropenem treatment”, “meropenem trials”, “meropenem tissue concentration”, meropenem CSF”, “meropenem meningitis”, and “meropenem drug-interaction”. In addition the book: The Pharmacological Basis of Therapeutics [1] has been consulted.
Efficacy and Safely of Meropenem
Thirty-two patients, aged 61+9 years, with infection of the lower respiratory-tract received initially 1 gram of ceftriaxone thrice-daily and this treatment did not extinguish the infection then the patients received meropenem intravenously at the daily dose of 1.5 grams. Meropenem resulted to be an effective and safe antibiotic as evidenced by bacterial culture and the adverse-effects were mild [2]. Forty-height patients, with a mean age of 50 years, had lower respiratory-tract infection and received meropenem intravenously at the daily dose of 3 grams. The plasma concentration of meropenem one hour after the administration of 1 gram of meropenem was 44.9+12.0 µg/ml. The minimum inhibitory concentration (MIC) of the organisms causing the infection ranged from 4 to 8 µg/ml thus this treatment effectively and safely cured the patients with lower respiratory-tract infection [3]. One-hundred-fifty-three patients were enrolled, 45 patients (29.4%) had urinary-tract and 108 patients (70.6%) had lower respiratory-tract infection. Patients with urinary-tract infection received meropenem intravenously at the dose of 0.5 grams thrice-daily and the patients with lower respiratory-tract infection received meropenem intravenously at the dose of 1 gram thrice-daily. The most common pathogens isolated were Escherichia coli, Streptococcus pneumoniae, and Pseudomonas aeruginosa. There were no relapses and the treatments were well-tolerated. Meropenem monotherapy is effective and safe empirical treatment of urinary-tract and lower respiratory-tract infections [4].
Meropenem was intravenously infused at the daily dose of 2.4 or 4.8 grams to 50 patients, aged 59 years (range, 18 to 91), who had a range of infections including lower respiratory-tract (N = 17), bone and/or joint (N = 14), and intraabdominal infections (N = 6), diabetic foot (N =4), urinary-tract infection (N = 3), otitis externa (N = 2), and other infections (N = 4). The most frequent pathogens isolated were Pseudomonas aeruginosa (N = 30), other gram-negative organisms (N = 8), Nocardia species (N = 2), and Burkholderia pseudomallei (N = 1) and meropenem effectively and safely extinguished the infections [5]. A total of 6,154 patients were enrolled and the most frequent diseases were skin, skin-structure, and intraabdominal infections, and bacterial meningitis. Meropenem was administered intravenously at the daily of 3 grams and was well-tolerated and effectively and safely treated the infections and bacterial meningitis [6]. Meropenem was administered intravenously at the daily dose of 3 grams to 50 patients with complicated skin, skin-structure, and complicated intraabdominal infections, and bacterial meningitis and this treatment effectively and safely cured the patients [7]. Meropenem was administered intravenously at the dose of 60 mg/kg thrice-daily to 30 paediatric cancer patients with a mean age of 7.5 years. Meropenem was well-tolerated and the efficacy as an empirical monotherapy in paediatric cancer patients with febrile neutropenia was satisfactory with a failure-rate of 23.3% on day 5 of treatment [8].
The use of prophylaxis with meropenem in patients undergoing allogeneic transplantation favourably affects the morbidity by reducing febrile episodes [9]. One-hundred-seventy-six patients with necrotizing pancreatitis were prospectively randomized to receive either prophylactic treatment with 0.5 grams of meropenem thrice-daily intravenously or 0.5 grams of imipenem thrice-daily intravenously. Meropenem is as effective as imipenem in preventing septic complications in patients with necrotizing pancreatitis [10]. Prophylactic efficacy of teicoplanin and meropenem against infections in open heart surgery was investigated in a retrospective observational study. The prophylactic agent combination of teicoplanin and meropenem prevents infections in open heart surgery [11]. A total of 538 patients, aged 47.4+15.6 years, undergoing colorectal surgery were enrolled and received either meropenem or cefoperazone intravenously. Of 538 patients only 67 patients (12.4%) developed surgical site infection. The surgical site infection occurred in 9.1% patients who received meropenem and in 19.8% patients who received cefoperazone (P-value < 0.001). Meropenem is superior to cefoperazone in prevention surgical site infection in patients undergoing elective colorectal surgery [12].
Sixty-two elderly patients, aged 86.6 years, with aspiration pneumonia were enrolled, 80.7% of patients were graded as "most severe", and all patients received meropenem intravenously at the daily dose of 1 gram. The overall clinical efficacy-rate was 61.3% and the mortality-rate was only 9.7%. Therapy of aspiration pneumonia with meropenem is clinically effective and tolerable in elderly patients [13]. Meropenem was administered intravenously at the daily dose of 3 grams to 31 elderly patients, aged 85 years, with aspiration pneumonia. The overall clinical efficacy-rate was 61.0% and the overall mortality-rate was only 10.1%. This treatment effectively cured elderly patients with aspiration pneumonia [14]. Twenty-five patient, aged 40 years, with nosocomial pneumonia caused by Acinetobacter baumannii (55%), Pseudomonas aeruginosa (27%), or by Streptococcus aureus (18%) received meropenem intravenously at the daily dose of 1.5 grams. At completion of treatment, 76.1% of patients were cured, 23.9% were improved, and the mortality-rate was only 12.0%. Meropenem was effective and well-tolerated in most patients [15]. Meropenem was administered intravenously at the dose of 1 gram thrice-daily to 111 patients with hospital acquired pneumonia or with ventilator-associated pneumonia. A satisfactory clinical response was observed in 68.3% of patients at the end of treatment and in 63.2% of patients at follow-up.
The overall satisfactory response-rate ranged from 65.3% to 100%. Meropenem was effective and well-tolerated in these patients [16]. It was compared the clinical outcomes of extended versus intermittent infusion of meropenem in treatment of nosocomial pneumonia. Two-hundred-fifty-height patents, aged 63.8+12.9 years, were enrolled, 161 patients (62.4%) underwent extended infusion, 97 patients (37.6%) underwent intermittent infusion, and meropenem was infused at the dose of 1 grams thrice-daily. The bacteria causing the pneumonia were Staphylococcus aureus, Klebsiella pneumoniae, Stenotrophomonas maltophilia, or Pseudomonas aeruginosa. At 14 days of treatment, 25 patients (15.5%) in the extended group and 22 patients (22.7%) in the intermittent group died (P-value = 0.174) and 141 patients (87.6%) undergoing the extended infusion and 81 patient (83.5%) undergoing intermittent infusion were cured (P-value = 0.185). An extended infusion of meropenem produces similar clinical outcomes as the intermittent infusion [17]. Meropenem was administered intravenously at the dose of 0.5 grams thrice-daily to 146 patients with complicated skin and soft-tissue infections. The clinical efficacy-rate ranged from 92.2% to 100% 7 to 14 days after the end of treatment. Meropenem was well-tolerated and effectively and safely treats these patients.
A higher dose of 1 gram thrice-daily of meropenem should be considered for treatment of complicated skin and soft-tissue infections caused by Pseudomonas aeruginosa [18]. It was assessed the efficacy of meropenem administered on a compassionate basis to 62 cystic fibrosis patients, aged 24+6 years, with chronic pulmonary infection. The patients had hypersensitivity reactions to β-lactam antibiotics and/or had infection caused by bacteria resistant to other antibiotics. Meropenem was intravenously infused at the dose of 2 grams thrice-daily for 14 days. Fifty-seven patients (91.9%) were chronically infected by Pseudomonas aeruginosa and 5 patients (8.1%) were chronically infected by Burkholderia cepacia. Meropenem proved to be a valuable drug for the treatment of these patients [19].
A double-blind, multicentre, randomized, controlled trial was conducted in 600 patients admitted to the intensive clinical unit with sepsis or with septic shock needing therapy with meropenem. Meropenem was continuously infused at the daily dose of 3 grams or an equal dose divided into three daily boluses (i.e. 1 gram thrice-daily) was infused. The primary endpoint was the outcome of reducing death and the secondary endpoint was the death from any cause at 90-days of treatment. Meropenem administered by continuous or intermittent infusion produces similar effects in critically ill patients with sepsis or with septic shock [20]. A clinical trial was conducted to compare the clinical and bacteriological efficacy of meropenem administered by a continuous infusion versus bolus in patients suffering from bacterial infections. Patients in the infusion group (N = 120) received a loading dose of 2 grams of meropenem followed by a continuous infusion of 4 grams daily of meropenem and patients in the bolus group (N = 120) received 2 grams of meropenem thrice-daily. Clinical cure at the end of therapy was comparable in both treatments. Bacteriological success-rate was higher in the infusion group as opposed to the bolus group (P-value = 0.020).
Multivariate logistic regression identified continuous infusion of meropenem as an independent predictor of better bacteriological efficacy (P-value = 0.040). Meropenem-related intensive care unit stay was shorter in the infusion group compared to the bolus group (P-value = 0.044). No severe adverse-effects related to meropenem were observed in both treatments. Continuous infusion and high intermittent dosage of meropenem are safe and infusion provides better bacteriological efficacy and a shorter care unit stay in critically ill patients [21]. A randomized controlled trial compared the efficacy of tigecycline versus that of meropenem in treatment of patients with postoperative complicated intraabdominal infections. A total of 56 patients were enrolled who were divided into 2 groups. One group included 30 patients who received meropenem and another group included 26 patients who received tigecycline. The two groups had similar demographic and clinical characteristics and had similar distribution of infecting microorganisms. Meropenem was administered intravenously at the dose of 1 gram thrice-daily and tigecycline was administered intravenously at an initial dose of 100 mg followed by 50 mg twice-daily. Clinical success-rate for meropenem and tigecycline at end of therapy was 73.3% and 76.9%, respectively, (P-value > 0.05).
Clinical success-rate at upon discharge visit was 76.7% for the meropenem group compared to 88.6% for the tigecycline group (P-value > 0.05). 60-Day all-cause mortality was 10.0% for meropenem and 11.54% for tigecycline (P-value > 0.05). The gastrointestinal disorders were the most frequently reported adverse-effects which were similar in meropenem and tigecycline groups (P-value > 0.05). Tigecycline had comparable activity as meropenem and both drugs were effective and well-tolerated therapy options in treating postoperative complicated intraabdominal infections [22].
Jaruratanasirikul, et al. [23] administered meropenem at the dose of 1 gram thrice-daily by 1 hour infusion to 14 patients with severe sepsis or with septic shock in the intensive care unit and in 14 healthy subjects. The patients and healthy subjects were aged > 18 years. The healthy subjects received a single dose of 1 gram of meropenem by 3 hours infusion. Table 1 summarizes the pharmacokinetics of meropenem in 14 patients with severe sepsis or with septic shock and in 14 healthy subjects. This table shows that the pharmacokinetic parameters of meropenem, except for the total body clearance, are altered in patients. The peak concentration and the trough concentration of meropenem, and the area under the concentration-time curve of meropenem are greater in patients than in healthy subjects and the elimination half-life of meropenem is longer in patients than in healthy subjects. In healthy subjects, meropenem is rapidly eliminated and the distribution volume of meropenem is lower that the water volume. In addition there is a remarkable variability in the pharmacokinetic parameters and this variability is accounted by the patient diseases.
Table 1: Pharmacokinetic parameters of meropenem which have been obtained in 14 patients with severe sepsis or with septic shock and in 14 healthy subjects. Patients received 1 gram of meropenem thrice-daily infused over 1 hour and healthy subjects received a single dose of 1 gram of meropenem infused over 3 hours. Values are the mean+SD, by Jaruratanasirikul, et al. [23].

Note: AUC = area under the concentration-time curve. *Student t test for unpaired data.
Hosmann, et al. [24] measured the concentration of meropenem in plasma, in brain, and in the cerebrospinal fluid. These authors administered 2 grams of meropenem thrice-daily by continuous infusion over 1 hour to 16 patients suffering from subarachnoid haemorrhage. Five patients received a single-dose of 2 grams of meropenem and 11 patients received 2 grams of meropenem thrice-daily for 14+9 doses. Table 2 summarizes the pharmacokinetic parameters of meropenem after-single administration and at the steady-state. This table shows that the area under the concentration-time curve of meropenem in brain is about 10% of that in plasma and the peak concentration of meropenem in brain is about 4% of that in plasma. The area under the concentration-time curve of meropenem in the cerebrospinal fluid and the peak concentration of meropenem in the cerebrospinal fluid are about 30% of those in plasma. In conclusion, meropenem poorly penetrates into the human brain and into the cerebrospinal fluid. Byl, et al. [25] collected specimens of lung, bronchial mucosa, and pleural tissue from 14 patients, aged 58 years (range, 38 to 68) who underwent lung surgery. Twelve patients underwent lobectomy or pneumonectomy and 2 patients underwent surgery to remove a bronchial cancer.
Table 2: Pharmacokinetic parameters of meropenem which have been obtained after the administration of a single-dose of meropenem and at steady-state. Values are the mean+SD, by Hosmann, et al. [24].
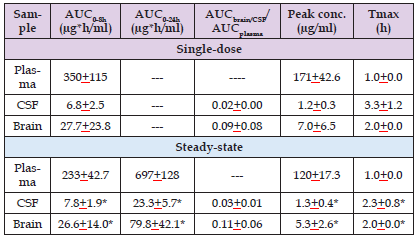
Note: AUC = area under the concentration-time curve. CSF = cerebrospinal fluid. Tmax = time to reach the peak concentration. *P-value < 0.05 differences compared with plasma values at steady-state (Wilcoxon signed-rank test).
The patients received 1 gram of meropenem infused over 3 min. The drug was injected approximately 1 (6 patients), 2 (4 patients), and 3 to 5 (4 patients) hours before the expected time of tissue sampling. The mean (range) of peak concentration of meropenem was 3.9 (0.2 to 8.2), 6.6 (3.0 to 13.3), and 2.8 (0.6 to 7.8) µg/gram in lung, bronchial mucosa, and pleural tissue, respectively, and these concentrations exceed the MIC of most respiratory pathogens. The penetration of meropenem into the respiratory-tract makes this drug a suitable agent for the treatment of bronchopulmonary infections.
Nau, et al. [26] enrolled 10 patients, aged 48 to 75 years, suffering from extra-cerebral infection caused by bacteria with proven or presumed susceptibility to meropenem. Serum creatinine concentration ranged from 0.7 to 1.7 mg/dl. Patients underwent external ventriculostomy due to occlusive hydrocephalus caused by cerebrovascular accidents and patients with clinical evidence of ventriculitis or with a creatinine concentration > 2 mg/dl were not considered. All patients received a first dose of 2 grams of meropenem by infusion over 30 min and the treatment was continued 16 hours then the patients received 1 gram of meropenem thrice-daily intravenously. Five patients had intracerebral haemorrhage, 2 patients had cerebral haemorrhage, 2 patients had subarachnoid haemorrhage, and 1 patient had infratentorial infarction. Table 3 summarizes the pharmacokinetic parameters of meropenem in serum and in CSF of 10 patients who received an infusion of 2 grams of meropenem. This table shows that the peak concentration of meropenem is 134 times higher in serum than in CSF, the area under the concentration-time curve of meropenem is 18 times higher in serum than in the CSF, and the elimination half-life of meropenem is 4.3 times longer in the CSF than in serum. In conclusion, meropenem poorly penetrates into the CSF and is eliminated slowly from the CSF.
Table 3: Pharmacokinetic parameters of meropenem in serum and in CSF of 10 patients who received an infusion of 2 grams of meropenem. Values are the mean+SD, by Nau, et al. [26].
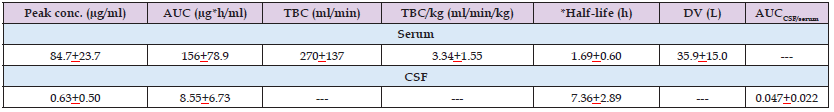
Note: AUC = area under the concentration-time curve. TBC = total body clearance. *Elimination half-life. DV = distribution volume.
Blassmann, et al. [27] administered meropenem at the dose of 2 grams thrice-daily as a prolonged infusion to 21 patients, aged 52 years (range, 46 to 80) and weighing 76 kg (range, 55 to 105) with ventriculitis. This dose was given to all patients except for those with drug adverse-effects or renal impairment (creatinine clearance ≥ 50 ml/min) for whom the dose was reduced to 1 gram thrice-daily. Table 4 summarizes the pharmacokinetic parameters of meropenem in the serum and CSF. This table shows that the distribution volume of meropenem is lower than the water volume and meropenem is rapidly eliminated in serum as the median elimination half-life is 0.63 hours. The area under the concentration-time curve is 13 times higher in serum than in the CSF, meropenem is slowly absorbed in the CSF as the median absorption half-life is 23.10 hours and is slowly eliminated from the CSF as the median elimination half-life of meropenem is 22 times longer in the CSF than in serum. In conclusion, meropenem poorly penetrates into the CSF and is slowly eliminated and absorbed from the CSF. Zhang, et al. [28] administered meropenem to 82 patients with post-neurosurgical meningitis who received meropenem intravenously according to a regimen of 2 grams thrice-daily, 1 gram thrice-daily, or 1 gram 4 times-daily.
Table 4: Pharmacokinetic parameters of meropenem which have been obtained in serum and in the CSF of 21 patients with ventriculitis. Meropenem was infused at the dose of 2 grams thrice-daily or was infused at the dose of 1 gram thrice-daily to patients with drug adverse-effects or with renal impairment (creatinine clearance ≥ 50 ml/min). Values are the median, the minimum, and the maximum, by Blassmann, et al. [27].

Note: TBC = total body clearance. DV = distribution volume. AUC = area under the concentration-time curve.
The peak concentration of meropenem in blood and in the CSF was 43.2+5.3 and 2.4+0.3 µg/ml, respectively, in patients who received 2 grams thrice-daily, 28.9+2.7 and 1.2+0.2 µg/ml, respectively, in patients who received 1 gram thrice-daily, and 31.5+3.4 and 1.6+0.2 µg/ml, respectively, in patients who received 1 gram 4 times-daily and the maximal percent penetration of meropenem into the CSF was 17.6%+7.3%, 14.3%+1.7%, and 30.9%+24.2%, respectively. Dosing regimens of meropenem of 1 gram 4 times-daily and 2 grams thrice-daily provide higher CSF penetration than 1 gram thrice-daily. A higher dose or a shorter dosing interval of meropenem may be more useful for clearance of pathogens.
Nicasio, et al. [29] administered meropenem at the dose of 2 grams thrice-daily by a 3 hours infusion to a female patient, aged 54 years, with meningitis caused by Serratia marcescens associated with an epidural abscess 57 days after surgery for a herniated spinal disk. The prolonged infusion regimen resulted in concentrations of meropenem in both serum and CSF above the MIC of 0.047 µg/ml for 100% of the dosing interval. After 6 days of therapy, the patient showed no further signs of infection and was subsequently discharged to a rehabilitation facility. At follow-up, she completed a 4 week course of the prolonged infused therapy without relapse or adverse-effects and the meningitis was cured. Schmutzhard [30] conducted two prospective randomised studies in 56 patients with bacterial meningitis who received either meropenem at a daily dose of 40 mg/kg infused over 8 hours up to a maximum of 6 grams daily (N = 28) or cefotaxime (N = 17) or ceftriaxone (N = 11). Clinical cure was observed in all 28 patients treated with meropenem (100%) and in 17 of the 22 patients (77.3%) treated with cephalosporins. All pre-treatment isolates were eradicated except for one isolate of Staphylococcus aureus in a patient treated with cefotaxime. Neurological sequelae were noted in 3 patients (10.7%) treated with meropenem and in 4 patients (14.3%) treated with cephalosporins (P-value > 0.05). No patients in either treatment group experienced seizures after the start of therapy.
Hearing impairment was recorded in 11 patients (39.3%) treated with meropenem and in 9 patients (32.1%) treated with cephalosporins (P-value > 0.05). Three patients in the meropenem group (10.7%) and 1 patient (3.6%) treated with cephalosporins died during treatment for reasons unrelated to therapy. This study indicates that meropenem is an effective and well-tolerated antibiotic for treatment of bacterial meningitis in adult patients. Klugman, et al. [31] investigated the effects of empirical treatment with meropenem compared to cefotaxime plus ampicillin in treating acute bacterial meningitis. Of 623 patients, 328 patients (52.6%) received cefotaxime plus ampicillin and 295 patients (46.7%) received meropenem. Using propensity score matching, the 30-day mortality-rate was 3.2% in patients who received cefotaxime plus ampicillin and was 3.6% in patients who received meropenem (P-value = 0.79) and the 90-day mortality-rate was 1.4% in patients who received cefotaxime plus ampicillin and 1.1% in patients who received meropenem (P-value = 0.62). These results indicate that meropenem is an effective empirical treatment option for adult patients with community-acquired acute bacterial meningitis. John, et al. [32] assessed the safely and efficacy of meropenem compared to those of cefotaxime in a prospective randomized trial of 190 children with bacterial meningitis. Seizures occurred within 24 hours before antibiotic therapy in 16 of 98 children (16.3%) who received meropenem and in 6 of 92 children (6.5%) who received cefotaxime (P-value < 0.05). Seizures during therapy occurred in 5 of 82 children (6.1%) who received meropenem and in 1 of 86 children (1.2%) receiving cefotaxime (P-value < 0.05). Bacterial eradication was found to be in 100% of children. These results indicate that meropenem is well-tolerated and is an effective treatment of bacterial meningitis in children.
The mechanisms of interaction of meropenem with valproic acid have been elucidated in rabbits and in rats. In rabbits, the total body clearance of valproic acid was increased 1.5 times by meropenem (6.09 and 4.28 ml/min/kg [P-value < 0.05] in presence and in absence of meropenem, respectively). The formation-rate of valproic acid-glucuronide was significantly increased (P-value < 0.05) to about 78% over control. The urinary excretion of valproic acid-glucuronide was significantly (P-value < 0.05) increased by meropenem due to the inhibition of hydrolysis of valproic acid-glucuronide. Thus the increase of the total body clearance of valproic acid caused by meropenem is due to an increase of renal clearance of valproic acid and by the suppression of hydrolysis of valproic acid-glucuronide [33]. In rats, meropenem causes a significant (P-value < 0.05) increase of accumulation of valproic acid into erythrocytes producing a significant decrease (P-value < 0.05) of plasma concentration of valproic acid [34]. Šíma, et al. [35] presented two case reports of drug interaction between valproic acid and meropenem. It was observed a 90.8% and 93.5% decrease in valproic acid serum concentration during concomitant administration of meropenem. Wen, et al. [36] observed a remarkable decrease in the plasma concentration of valproic acid when the drug was used concomitantly with meropenem.
Meropenem was administered at the daily dose of 1.2 grams and valproic acid was administered at the daily dose of 1.6 grams. The plasma concentration of valproic acid was 67.3+4.6 and 15.3+1.6 μg/ml (P-value < 0.001, N = 21) in absence and in presence of meropenem, respectively. Spriet, et al. [37] performed a retrospective study of 18 month period to assess the extent and clinical impact of meropenem on valproic acid. Thirty-nine patients were treated simultaneously with valproic acid and meropenem. The pharmacokinetic interaction was observed in all 39 patients, with an average drop in valproate plasma concentrations of 66% and the decrease occurred within 24 hours after start of treatment. This interaction was clinically relevant with electro-clinical deterioration in 55% of patients. To avoid patients’ possible neurologic deterioration, meropenem and valproate should not be administered together. Suntimaleeworakul, et al. [38] administered 2.4 grams of valproic acid to a 77-year-old-male patient and the serum concentration of valproic acid was 66.5 µg/ml after 61 days of treatment. He developed fever and was treated with 1.5 grams of meropenem and the serum concentration of valproic acid decreased to 18.9 µg/ml one day after meropenem administration.
Meropenem is a carbapenem antibiotic, is a β-lactam antibiotic, has a broader spectrum of activity than most other β-lactam antibiotics being active against gram-positive and gram-negative organisms, is resistant to β-lactamases, and is available for intravenous administration. The dose of meropenem is 1 to 2 grams thrice-daily infused over 3 hours and meropenem is employed for hospital-onset of respiratory, gastrointestinal, and urinary-tract infections [1]. The efficacy and safely of meropenem has been reviewed. Meropenem administered intravenously at the daily dose of 1.5 grams effectively and safely treats patients with lower respiratory-tract infection [2]. Meropenem was administered intravenously at the daily dose of 3 grams to patients with lower respiratory-tract infection. Following the intravenous dose of 1 gram of meropenem the plasma concentration of meropenem is 44.9+12.0 µg/ml and the MIC of the organisms causing the infection ranges from 4 to 8 µg/ml thus this treatment effectively and safely cures the patients [3]. Patients with urinary-tract infection received meropenem intravenously at the dose of 0.5 grams thrice-daily and patients with lower respiratory-tract infection received meropenem intravenously at the dose of 1 gram thrice-daily. The most common pathogens isolated were Escherichia coli, Streptococcus pneumoniae, and Pseudomonas aeruginosa and meropenem effectively and safely treats these patients [4].
Meropenem was intravenously infused at the daily dose of 2.4 to 4.8 grams to patients with lower respiratory-tract, bone and/or joint, and intraabdominal infections, diabetic foot, urinary-tract infection, otitis externa, and other infections. The most common organisms isolated were Pseudomonas aeruginosa and other gram-negative organisms, Nocardia species, and Burkholderia pseudomallei and this treatment extinguishes the infections [5]. Meropenem was administered intravenously at the daily dose of 3 grams to patients with skin, skin-structure, and intraabdominal infections and with bacterial meningitis and this treatment is well-tolerated and effectively and safely cures the patients [6], meropenem administered intravenously at the daily dose of 3 grams effectively and safely treats skin, skin-structure, complicated intraabdominal infections, and bacterial meningitis [7], and meropenem was administered intravenously at the dose of 60 mg/kg thrice-daily to 30 paediatric cancer patients with a mean age of 7.5 years. Meropenem is well-tolerated and the efficacy as an empirical monotherapy in paediatric cancer patients with febrile neutropenia is satisfactory with a failure-rate of 23.3% on day 5 of treatment [8].
The prophylaxis with meropenem has been reviewed. The prophylaxis with meropenem in patients undergoing allogenic transplantation reduces febrile episodes [9], meropenem or imipenem was administered intravenously at the dose of 0.5 grams thrice-daily and meropenem is as effective as imipenem in preventing septic complications in patients with necrotizing pancreatitis [10], the prophylaxis with teicoplanin co-administered with meropenem prevents infections in open heart surgery [11], and meropenem is superior to cefoperazone (P-value < 0.001) in preventing surgical site infections in patients undergoing colorectal surgery [12]. The treatment of bacterial infections with meropenem has been reviewed. The therapy of aspiration pneumonia with meropenem administered intravenously at the daily dose of 1 gram is clinically effective and tolerable in elderly patients [13], meropenem administered intravenously at the daily dose of 3 grams effectively treats elderly patients with aspiration pneumonia [14], patients with nosocomial pneumonia caused by Acinetobacter baumannii, Pseudomonas aeruginosa, or by Streptococcus aureus received meropenem intravenously at the daily dose of 1.5 grams.
At completion of treatment, 76.1% of patients were cured, 23.9% were improved, and the mortality-rate is only 12.0% thus meropenem is effective and well-tolerated in most patients [15], meropenem was administered intravenously at the dose of 1 gram thrice-daily to patients with hospital acquired pneumonia or with ventilator-associated pneumonia and this treatment is effective and well-tolerated and the overall satisfactory response-rate ranges from 65.3% to 100% [16], patients with nosocomial pneumonia received meropenem at the dose of 1 gram thrice-daily and meropenem was administered by extended or by intermittent infusion. The bacteria causing the pneumonia were Staphylococcus aureus, Klebsiella pneumonia, Stenotrophomonas maltophilia, or Pseudomonas aeruginosa and the extended infusion of meropenem produces similar clinical outcomes as the intermittent infusion and the mortality-rate is similar in two infusion procedures [17], meropenem was administered intravenously at the dose of 0.5 grams thrice-daily to patients with complicated skin and soft-tissue infections and this treatment is well-tolerated and effectively and safely cures the patients. A higher dose of 1 gram thrice-daily of meropenem should be considered for treatment of complicated skin and soft-tissue infections caused by Pseudomonas aeruginosa [18], meropenem was intravenously infused at the dose of 2 grams thrice-daily for 14 days to cystic fibrosis patients with chronic pulmonary infection caused by Pseudomonas aeruginosa or by Burkholderia cepacia and meropenem treats these patients [19].
The trials conducted with meropenem have been reviewed. A clinical trial was conducted in patients with sepsis or with septic shock who needed therapy with meropenem. Meropenem was continuously infused at the daily dose of 3 grams or an equal dose divided in three daily boluses (i.e. 1 gram thrice-daily) was infused. The primary endpoint was reducing the death and the secondary endpoint was the death from any cause at 90-days. Continuous and intermittent infusion of meropenem produces similar effects in critically ill patients with sepsis or with septic shock [20]. A clinical trial compared the clinical and bacteriological efficacy of continuous infusion versus bolus administration of meropenem. Infusion consisted in 2 grams of meropenem followed by a continuous infusion of 4 grams daily of meropenem and the bolus consisted in 2 grams thrice-daily of meropenem. Clinical cure is similar in both treatments whereas the bacteriological efficacy is higher (P-value = 0.020) in the infusion than in the bolus administration. Meropenem-related intensive care unit stay is shorter in patients who received meropenem by infusion than in patients who received meropenem by bolus (P-value = 0.044). Continuous infusion and high intermittent dosage of meropenem are safe and infusion provides better bacteriological efficacy and a shorter care unit stay in critically ill patients [21].
A clinical trial was conducted to compare the efficacy of tigecycline versus that of meropenem in treatment of patients with postoperative complicated intraabdominal infections. Meropenem was administered intravenously at the dose of 1 gram thrice-daily and tigecycline was administered intravenously at an initial dose of 100 mg followed by 50 mg twice-daily. The clinical success and the mortality at 60-day all-cause are similar in both treatments. Tigecycline has comparable activity as meropenem and both drugs are effective and well-tolerated therapy options in treating postoperative complicated intraabdominal infections [22]. Jaruratanasirikul, et al. [23] studied the pharmacokinetics of meropenem in 14 patients with severe sepsis or with septic shock and in 14 healthy subjects. Patients received 1 gram of meropenem thrice-daily infused over 1 hour and the healthy subjects received a single dose of 1 gram of meropenem infused over 3 hours. The elimination half-life of meropenem is 3.30+3.45 and 0.61+0.14 hours (P-value < 0.05) in patients and in healthy subjects, respectively. All pharmacokinetic parameters, except for the total body clearance, are altered in patients. The concentration of meropenem in human plasma and tissues has been reviewed. Following the administration of 2 grams thrice-daily of meropenem by continuous infusion meropenem poorly penetrates into the cerebrospinal fluid and into the brain and the peak concentration of meropenem in plasma, cerebrospinal fluid, and in brain is 171+42.6, 1.2+0.3, and 7.0+6.5 µg/ml, respectively [24].
Meropenem was infused at the dose of 1 gram to 14 patients undergoing lung surgery and the mean peak concentration of meropenem is 3.9, 6.6, and 2.8 µg/gram in human lung, bronchial mucosa, and pleural tissue, respectively. These concentrations are higher the MIC of most respiratory pathogens thus meropenem is a suitable antibiotic to tract bacterial infections of the respiratory-tract [25]. The penetration of meropenem into the cerebrospinal fluid has been reviewed. Nau, et al. [26] studied the pharmacokinetics of meropenem in serum and in the cerebrospinal fluid. Meropenem was first infused at the dose of 2 grams and then a dose of 1 gram thrice-daily of meropenem was administered. The peak concentration, the area under the concentration-time curve, and the elimination half-life of meropenem are 84.7+23.7 µg/ml, 156+78.9 µg*h/ml, and 1.69+0.60 hours, respectively, in serum and are 0.63+0.50 µg/ml, 8.55+6.73 µg*h/ml, and 7.36+2.89 hours, respectively, in the cerebrospinal fluid. These results indicate that meropenem poorly penetrates into the cerebrospinal fluid and is eliminated slowly from the cerebrospinal fluid. Blassman, et al. [27] studied the pharmacokinetics of meropenem in serum and in the cerebrospinal fluid of patients. Meropenem was infused at the dose of 2 grams thrice-daily and patients with drug adverse-effects or with renal impairment received meropenem intravenously at the dose of 1 gram thrice-daily.
The median elimination half-life and the median area under the concentration-time curve of meropenem are 0.63 hours and 350 µg*h/ml, respectively, in serum and are 13.86 hours and 26.56 µg*h/ml, respectively, in the cerebrospinal fluid and the media absorption half-life of meropenem is 23.10 hours in the cerebrospinal fluid. These results indicate that meropenem is slowly eliminated from the cerebrospinal fluid, poorly penetrates into the cerebrospinal fluid, and is slowly absorbed from the cerebrospinal fluid. Zhang, et al. [28] administered meropenem to patients with post-neurosurgical meningitis. Meropenem was administered intravenously at the dose of 2 grams thrice-daily, 1 gram thrice-daily, or 1 gram 4 times-daily. Following the administration of meropenem at the dose of 2 grams thrice-daily the concentration of meropenem in the blood and in the cerebrospinal fluid is 43.2+5.3 and 2.4+0.3 µg/ml, respectively. Following the administration of meropenem at the dose of 1 gram thrice-daily the concentration of meropenem in the blood and in the cerebrospinal fluid is 28.9+2.7 and 1.2+0.2 µg/ml, respectively and following the administration of meropenem at the dose of 1 gram 4 times-daily the concentration of meropenem in blood and in the cerebrospinal fluid is 31.5+31 and 1.6+0.2 µg/ml, respectively. Dosing regimens of meropenem of 1 gram 4 times-daily and 2 grams thrice-daily provide higher penetration of meropenem into the cerebrospinal fluid than 1 gram thrice-daily.
A higher dose and shorter dosing interval of meropenem may be more useful for clearance of pathogens. These results indicate that meropenem poorly penetrates into the cerebrospinal fluid. The treatment of bacterial meningitis with meropenem has been reviewed. Nicasio, et al. [29] infused meropenem at a dose of 2 grams thrice-daily to a female patient with the meningitis caused by Serratia marcescens and the concentration of meropenem in both serum and in the cerebrospinal fluid is above the MIC of 0.047 µg/ml for 100% of the dosing interval and after 4 week course of therapy the meningitis is cured. Schmutzhard, et al. [30] infused meropenem up to a maximum of 6 grams (N = 28) or cefotaxime (N = 17) or ceftriaxone (N = 11) to patients with bacterial meningitis. The clinical cure is observed in 100% of patients treated with meropenem and in 77.3% of patients who received the cephalosporins. Neurological sequelae occur in 10.7% of patients who received meropenem and in 14.3% of patients treated with cephalosporins (P-value > 0.05). No patients experienced seizures and hearing impairment occurs in 39.3% of patients treated with meropenem and in 32.1% of patients who received cephalosporins (P-value > 0.05). Meropenem is an effective and well-tolerated antibiotic for treatment of bacterial meningitis in adult patients.
Klugman, et al. [31] administered meropenem or cefotaxime plus ampicillin to patients with acute bacterial meningitis. The 30-day mortality-rate is 3.2% in patients who received cefotaxime plus ampicillin and is 3.6% in patients who received meropenem (P-value = 0.79) and the 90-day mortality-rate is 1.4% in patients who received cefotaxime plus ampicillin and 1.1% in patients who received meropenem (P-value = 0.62). Meropenem is an effective treatment of adult patients with community-acquired acute bacterial meningitis. John, et al. [32] assessed the safely and the efficacy of meropenem compared to those of cefotaxime in children with bacterial meningitis. Seizures during therapy occur in 6.5% of children who received meropenem and in 1.2% of children who received cefotaxime (P-value < 0.05). The bacterial eradication is found to be 100% in both groups of children. Meropenem is well-tolerated and is an effective treatment of bacterial meningitis in children. The interaction of meropenem with valproic acid has been reviewed. The mechanisms of interaction of meropenem with valproic acid have been elucidated in rabbits and in rats. In rabbits, the total body clearance of valproic acids is increased 1.5 times by meropenem, the formation-rate of valproic acid-glucuronide in significantly increased (P-value < 0.05) by meropenem, and the urinary excretion of valproic acid-glucuronide is significantly increased (P-value < 0.05) by meropenem due to the inhibition of hydrolysis of valproic acid-glucuronide.
Thus the increase of the total body clearance of valproic acid caused by meropenem is due to an increase of renal clearance of valproic acid and by the suppression of hydrolysis of valproic acid-glucuronide [33]. In rats, meropenem causes a significant increase (P-value < 0.05) of accumulation of valproic acid into erythrocytes producing a significant (P-value < 0.05) decrease of plasma concentration of valproic acid [34]. Šíma, et al. [35] presented two case reports of drug interaction between valproic acid and meropenem. It was observed a 90.8% and 93.5% decrease in valproic acid serum concentration during concomitant administration of meropenem. Meropenem was administered at the daily dose of 1.2 grams and valproic acid was administered at the daily dose of 1.6 grams and the plasma concentration of valproic acid is 67.3+4.6 and 15.3+1.6 (P-value < 0.001) in absence and in presence of meropenem, respectively [36]. Spriet, et al. [37] performed a retrospective study of 18 month period and observed that meropenem causes a 66% decrease of valproic acid concentration in plasma and this decrease occurs 24 hours after start of treatment. Following the administration of 2.4 grams of valproic acid to an old patient the serum concentration of valproic acid is 66.5 µg/ml and following the administration of 1.5 grams of meropenem the serum concentration of valproic acid decreases to 18.9 µg/ml one day after meropenem administration [38].
In conclusion, meropenem is a carbapenem antibiotic which has a broad spectrum of activity being active against gram-positive and gram-negative organisms. Meropenem is available for intravenous administration and is usually administered at a dose of 1 to 2 grams thrice-daily by extended infusion. Meropenem is employed for hospital-onset of the respiratory, gastrointestinal, and urinary-tract infections and meropenem has been found efficacy and safe. The prophylaxis with meropenem, the treatment of bacterial infections with meropenem, and the trials conducted with meropenem have been reviewed. The pharmacokinetics of meropenem have been studied in patients with severe sepsis or with septic shock and in healthy volunteers and the mean elimination half-life of meropenem is 3.30 and 0.61 hours (P-value < 0.05) in patients and in healthy volunteers, respectively. All pharmacokinetic parameters, except for the total body clearance, are altered in patients. The concentration of meropenem in human plasma and tissues has been reviewed. The penetration of meropenem into the cerebrospinal fluid has been reviewed and meropenem poorly penetrates into the cerebrospinal fluid however meropenem treats bacterial meningitis. The interaction of meropenem with valproic acid has been reviewed and meropenem significantly decreases the plasma concentration of valproic acid. The aim of this study is to review the clinical pharmacology of meropenem.
The author declares no conflicts of financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employments, gifts, and honoraria. This article is a review and drugs have not been administered to men or animals
The author thanks Dr. Patrizia Ciucci and Dr. Francesco Varricchio, of the Medical Library of the University of Pisa, for retrieving the scientific literature.
The author conceived, wrote and typed the present manuscript. Prof. Gian Maria Pacifici, via Sant’Andrea 32, 56127 Pisa, Italy, is the corresponding author. The author is responsible for the reported research. He conceived and designed the study, executed the analysis, interpreted the results, and he drafted, revised, and approved the manuscript as submitted. The present article is a review and drugs have not been administered to men or animals.


