Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Huma Rana1, Amar Prakash Garg2* and Sourabh Jain3
Received: February 07, 2024; Published: February 23, 2024
*Corresponding author: Amar Prakash Garg, Dean Academics and Director Research, Swami Vivekanand Subharti University, NH-58, Subhartipuram, Meerut-250005, India
DOI: 10.26717/BJSTR.2024.55.008666
Black pepper and turmeric are commonly used as antimicrobial food additives as they have numerous health benefits besides their known antimicrobial properties. The edible coatings containing antimicrobial spices may extend the shelf life of Jaggery by providing a semi-permeable barrier to gases and water vapor and thus preventing it from deterioration. Turmeric (TC) and black pepper coated (PC) Jaggery were evaluated for physico-chemical properties, antioxidant activity, total viable count and antimicrobial activity and compared with non-coated control. Physico-chemical analysis revealed no significant difference between non-coated and coated Jaggery, and the phenolics, tannins and flavonoids were preserved better in coated samples. Edible Coating of Jaggery samples revealed significantly lesser (p≤0.01) microbial counts in comparison to non-coated. DPPH radical scavenging ability and reducing power potential of coated Jaggery exhibited better anti-oxidant activity in comparison to control. Turmeric and black pepper coatings were effective in inhibiting the growth of Gram’s positive as Gram’s negative bacteria when compared with untreated Jaggery. The results suggest that turmeric and black pepper coatings of Jaggery may be used to enhance the shelf life of Jaggery to maintain its quality as comparable to fresh Jaggery.
Jaggery is the common agriculture-based cottage industry of western Uttar Pradesh and farmers are compelled to sell their product when fresh but at lower price. Hence, it was felt desirable to develop better methods of storage to enhance its shelf life. Antimicrobial activity of black pepper and turmeric coatings were determined using agar double diffusion method and the physioco-chemical characteristics were determined using standard protocol for determining reducing sugars, proteins, phenols and flavonoids. The experiments revealed that the edible coatings with turmeric and black pepper enhanced the shelf life of Jaggery without any significant microbial spoilage. The coatings with spices also preserved the contents of phenolics, flavonoids and tannins resulting in better anti-oxidant activity in comparison to untreated control. The content of reducing sugars in edible coating with turmeric and black pepper were also preserved better. The edible coatings containing black pepper and turmeric enhanced the shelf life of Jageery for 6 months.
Keywords: Jaggery; Edible Coating; Turmeric; Black Pepper; Anti-Oxidant Activity; Antimicrobial Activity
Abbreviations: AW: Activity, NCJ: Non-Coated Jaggery, PDA: Potato Dextrose Agar, NAM: Nutrient Agar Medium, GAE; Gallic Acid Equivalent, PC: Pepper Coated, TC: Turmeric Coated, CMC: Carboxy Methyl Cellulose, NC: Non-Coated
Jaggery, a plant product high in sugar that is used all over the world, is traditionally made by condensing sugarcane (Saccharum officinarum) juice. Jaggery is very beneficial nutritionally and medicinally. Jaggery is regarded as a therapeutic sugar in Indian Ayurvedic medicine, used to heal lung and throat infections. In vivo studies reported that a dietary supplement of Jaggery was found to exhibit health benefits [1] Jaggery being a least processed sugar retains phenolics and other phytochemicals with potent biological activities like antioxidant, cytoprotective and anthelmintic activity [2]. The physical and chemical composition of Jaggery as well as its storage environment play a significant role in determining the quality of product. During storage, solid Jaggery undergoes liquefaction and changed to dark color, which is due to absorption of moisture and microbial attack [3,4]. Physically, it dissolves and liquefies, disturbing the texture. It also dilutes the sugars and lowers the sweetness. Chemically, it promotes inversion of sucrose which in turn leads to loss of texture, structure and body hardness. Moisture gain also encourages microbial spoilage and degradation resulting in lowering of quality and reduced economic value [5]. To prevent microbial deterioration, edible films or coatings are applied to the food. An ideal edible coating is a thin layer of material that can be consumed and provides a barrier to oxygen, external microbes, moisture, and prevent solute movement from food [6,7]. It can also reduce decay without affecting the quality of fresh fruits and vegetables and extend their storage life without causing anaerobiosis [8]. The preservative effect of black pepper (Piper nigrum) and Turmeric (Curcuma Longa L.) extracts on Jaggery have earlier been reported [9,10]. Turmeric belonging to the family of Zingiberaceae, has main biochemical compounds curcumin and curcuminoids commonly used as spices and coloring agents in food. Turmeric has also anti-inflammatory, antifungal, antimicrobial, antioxidant and anti-proliferative properties [10,11]. Traditional antibacterial remedies make considerable use of black pepper (Piper nigrum). There are several recognized piperidine and pyrrolidine alkamides found in P. nigrum. It is well known that the main bioactive component of black pepper, piperine, has antibacterial qualities [9]. Hence, the present investigation is undertaken to evaluate physico-chemical properties, microbial characteristics, antioxidant activity and antibacterial activity of carboxy methyl cellulose (CMC) based black pepper and Turmeric coatings on Jaggery.
Sample Collection and Materials
Fresh Jaggery samples (prepared from sugarcane variety ‘Co 0238’) were procured from local small scale Jaggery manufacturing unit situated at Muzaffarnagar, India. Black pepper liquid extract (Piper nigrum) (BRM chemicals, India) and turmeric (Curcuma longa L.) water extracts (95%) (Purenso, India) were purchased from local pharmacy store. Food-grade CMC (99.9%) with an average molecular weight of 41,000 g.mol-1, Glycerol (analytical grade) and other reagents were purchased from Himedia Laboratories, Mumbai, India. Initial analysis of Jaggery was carried out in April 2023 and later after six months of storage in September 2023.
Edible Coating Preparation and Sample Storage
Carboxy methyl Cellulose (1.5 g) was dissolved in 100 mL distilled water and stirred at temperature of 75°C until the mixture became clear and 5% (w/v) glycerol was added to it as plasticizer. Thereafter the solutions were cooled to 50°C and antimicrobial agents (black pepper and turmeric extracts) were at 2% (w/v) concentration were added with constant stirring [12]. Three types of coating solution were prepared: turmeric coated (TC), black pepper coated (PC) and turmeric plus black pepper (equal concentration) coated (TPC) and the control included with no coatings. Jaggery samples (100 g cubes) were coated by dipping them into the pre-prepared coating solutions for 120 s at room temperature, then drying for 60s at room temperature under blowing filtered air under Laminar Air Flow. Both non-coated (NC) and coated samples (TC, PC, TPC) were stored in aluminium pouches for six months for further analysis.
Physico-Chemical Characterization
Both coated and non-coated stored Jaggery samples were subjected to further analysis viz. Physico-chemical characterization (pH, color, turbidity, filterability, insoluble solids, water activity) as described by Guerra and Mujica [13]. Samples were dissolved in sterile distilled water and the pH was determined using digital pH meter (Labman, India). Color (5% w/v solution of Jaggery samples) was determined by measuring OD at 540 nm using visible spectrophotometer (Labman, India). The water activity was measured by placing the samples in water activity meter (Labtron, India) and by measuring the equilibrium relative humidity. Turbidity (5% w/v solution of Jaggery samples) was determined by measuring transmittance at 740 nm using visible spectrophotometer. Filterability (%) was calculated using the ratio of filtered volumes of 100mL each of sucrose (280 Brix) solution and 5 % w/v Jaggery sample solution when filtered through filter paper for 3 min. Insoluble solids were determined by drying and weighing the residue left on filter paper after filtering 1g of Jaggery sample solution.
Moisture, protein, ash, reducing sugars, sucrose content of Jaggery were determined following the methods of Official AOAC [14]. Moisture content (%) was determined as weight ratio expressed after drying 1g of Jaggery sample for 24h in hot air oven. Reducing sugars (%) was determined by using titrating Jaggery sample solution with known volume of Fehling solution. Sucrose (%) was also determined by similar titration method, but after the inversion of sample solution with acid followed by neutralization with alkali. Ash content was determined by incinerating 10g of Jaggery sample in muffle furnace and comparing the weight with air-dried Jaggery sample. For protein content measurements, 100 μL sample solution and 5 mL Bradford reagent were mixed and incubated for 5 min. Absorbance at 595 nm was recorded and plotted with standard curve of bovine serum albumin.
Phyto-Chemical Characterization
The total flavonoids content, tannins content, saponins, total alkaloid and total phenol content of Jaggery were determined using aluminium chloride method [15] and Folin–Ciocalteu’s method [16], respectively. For total phenol content, 2 ml of Folin-Ciocalteu reagent and 2 ml of 10% sodium bicarbonate solution were mixed together with 500 µl of the sample and incubated for 1h at room temperature. Absorbance was recorded at 765 nm. Gallic acid was used as standard and total phenol content was expressed as mg gallic acid equivalent (GAE)/ gram of sample. For total flavonoids, reaction mixture was prepared by adding 5ml of 10% aluminium chloride solution with 5ml of sample solution and absorbance at 415 nm was recorded after incubation for 30 min at room temperature. Catechin is used as standard and total flavonoid content is expressed as mg catechin per gram of sample (mg/g). For total alkaloids, reaction mix was prepared by adding 100 µl of sample with 40ml of 10% acetic acid in ethanol and incubated for 4h at room temperature. After that, ammonium hydroxide was added drop wise to the mix and residue was allowed to settle down for 1h. The residue was than filtered, dried and weighed. Total tannin content was determined by preparing reaction mix of 1 ml sample with 7.5 ml distilled water, 0.5 ml of Folin-Ciocalteu reagent and 1 ml of 35% solution of sodium carbonate. After 1h, absorbance was recorded at 760nm. Total tannin content is expressed as tannic acid equivalent in mg/ gram of sample. Saponins were determined by purifying 5 ml of sample solution with ethanol and Di-ethyl ether and concentrating with n-butanol.
Anti-Oxidant Activity
The Jaggery's capacity to scavenge DPPH radicals was assessed using the methodology outlined by Yamaguchi, et al. [17]. 1 ml sample solution was mixed with standard BHT at varying concentrations. 3ml of DPPH was added to the mix and incubated for 30 min in dark. Absorbance was recorded at 517nm and DPPH radical scavenging was represented as I %= (A control-A sample)/A control * 100. The effective concentration (EC50) for DPPH (50%) was also calculated. Further, reducing power of Jaggery was determined according to the method reported earlier by Yen and Chen [18]. Different concentration of Jaggery sample solution was mixed with standard antioxidant Trolox and equal volume of 0.2M phosphate buffer and 1% potassium ferricyanide was added. The reaction mix was incubated at 50℃ for 30 min. Further, mix was centrifuged at 3000 rpm after adding 10% trichloro acetic acid. Supernatant was collected and mixed with 1% ferric chloride solution and sterile water. Absorbance was recorded at 700 nm.
Microbiological and Statistical Analysis
For microbiological analysis, Standard Plate Count, Yeast, and Mould count were made using Nutrient Agar medium (NAM) for bacteria and Potato Dextrose Agar (PDA) for mould, while yeasts were isolated using Yeast Agar medium [9]. Data were subjected to statistical analysis for testing their significance by employing Analysis of Variance (ANOVA) technique.
Physico-Chemical Characterization of Coated Jaggery
The results of physical properties of non-coated and coated Jaggery are represented in Table 1. The pH of coated Jaggery and non-coated Jaggery were in the range of 5.7–5.9 that is in accordance with Guerra and Mujica [13]. Low pH of Jaggery may be due to deficiency of lime added at the time of juice clarification. Color of Jaggery finds to be the primary factor for consumer preference and market, and is dependent on dark compounds formed during processing. Browning of Jaggery can be caused by the Maillard reaction, oxidation of phenolic chemicals, alkaline breakdown of sucrose, or caramelization of sugars. [19]. Coated Jaggery depicts elevated absorbance at 540 nm (TPC>TC>PC) compared to non-coated Jaggery (NC). NC has golden brown color, while, darkened color was resulted in all coated Jaggery samples. Moisture content and water activity are two important parameters determining the quality, stability and shelf-life of foods during storage. TPC and PC showed a marked increase (0.9 %) in moisture content but a very slight increase in moisture content observed for TC. This shows that coating of Jaggery samples helped in retaining moisture content up to some extent. Water activity (aw) represents the water status in the food system and governs microbial growth [20]. Coating of Jaggery samples showed significant (p≤0.01) differences in water activity as noted by marked decreasing trend in values obtained for non-coated and coated samples. The results indicated that TPC and PC could offer better shelf-life and promising quality for Jaggery during storage. However, aw in the range 0.60–0.65 finds to be the optimum condition for growth of osmophilic and xerophilic microbes such as Aspergillus, Saccharomyces and thus supports their growth on Jaggery and results in spoilage [20].
Turbidity of all coated Jaggery showed a gradual increase (TPC<PC<TC) with respect to NC Jaggery. About 8–9 %, increase in turbidity was observed between NC compared to PC and TC, respectively. Marginal increase in filterability is seen between NC and TC Jaggery. However, results showed initial remarkable increased (6 and 15 %) filterability in PC and TPC, respectively, but the ash content was differed by 0.01 % in all coated Jaggery.
The results of chemical properties are represented in Table 1. Sucrose and reducing sugar content of coated Jaggery showed very marginal increase in coated Jaggery (TPC>TC>PC). Protein content TC and PC showed no significant difference over NC Jaggery, but, TPC depicted increase of about 0.4 mg/g of protein content. Increase in total phenol, tannin and flavonoid contents was resulted in all coated Jaggery (TPC>PC>TC). TC, PC and TPC exhibited increase in 11.1, 12.0 and 16.5 % phenol; 15.4, 14.6 and 16.2 % tannin and 10.6, 6.7 and 7.7 % flavonoid contents, respectively from NC Jaggery. Because of their distinct functional groups, flavonoids are the most prevalent form of dietary polyphenols with antioxidant potential. Both flavonoids and total phenols are in accordance with the previously reported study by Ahmad et. al [21]. Thus, our results depicted that edible coated Jaggery may be used as a source of antioxidants. Saponin content and total alkaloid content values were not significantly different between samples and depicted only marginal increase in coated samples a compared to non-coated.
Antioxidant Activity
Antioxidant activity of coated Jaggery was measured by two in vitro assays, i.e., DPPH radical scavenging ability and reducing power assay. DPPH is a stable free radical, and in its radical form absorbs at 517 nm whose absorption decreases after acceptance of an electron or hydrogen atom from an antioxidant due to the formation of its non-radical form DPPH-H [22]. The degree of decolorization of DPPH is a stoichiometric measure of the antioxidant potential of test samples. The scavenging ability of TPC, TC and PC were expressed in terms of EC50 values as shown in Table 2. All coated Jaggery showed concentration dependent free radical scavenging activity. Coated Jaggery decreased EC50 concentration than non-coated Jaggery. TC, PC and TPC had EC50 of 3.098, 3.076 and 3.038 mg/mL, respectively. EC50 of BHT, used as standard was 0.0075 mg/mL. Both coated and non-coated Jaggery showed higher (450 folds) EC50 concentration than standard BHT. Results of DPPH radical assay showed a positive correlation (r = 0.92, 0.87 and 0.88) with total phenolics of TC, PC and TPC Jaggery, respectively. High correlations between total phenolics and scavenging of DPPH radical indicated that polyphenols present in the coated Jaggery are the main antioxidants. Further, reducing capacity assay provides a measure of compound’s ability to donate electrons and reduce the oxidized intermediates formed in peroxidation process. The assay is based on the reduction of Fe+3-ferricyanide complex that is monitored at 700 nm. A rise in absorbance signifies a rise in reductive capacity [23].
Table 2: DPPH radical scavenging activity (standard BHT) and reducing power (standard Trolox) of non-coated and coated Jaggery.

Since reducing power of a compound serves as a significant indicator of its antioxidant activity [24], coated Jaggery assayed for reducing power ability. In Table 2, coated Jaggery exhibited in-vitro ferric reducing potential in increasing manner. The absorbance of coated Jaggery at 700 nm had increased than non-coated Jaggery. Trolox, used as a standard showed absorbance of 1.37 at 50 lg/mL. The reducing potential of TC, PC and TPC increased by 23.22, 26.00 and 24.53 %, respectively than non-coated Jaggery. Natural antioxidants play an important role in the prevention and interception of oxidative damage and have great impact on the safety and acceptability of the food system. They keep the food stable against oxidation and act as a potent preservative by controlling microbial growth. Antioxidants have many health benefits, including preserving biological function and guarding against diseases like cirrhosis, diabetes, heart disease, gastropathy, chronic renal disease, and cancers [25]. In addition, antioxidant activity of plant is often associated with polyphenols that with hydrogen donating capacity inhibits free radical induced oxidation [18]. The phenolic compounds of sugarcane juice exhibited antioxidant potential [26] and conferred various biological activities. The antioxidant compounds extracted from Jaggery showed stronger antioxidant potential than BHT in earlier reports [27].
The studies on the in vitro stability and antioxidant capacity showed that when curcumin is embedded in the O/W SFME, its stability and antioxidant activity are significantly improved [28]. It is also reported that naturally occurring antioxidants found in black pepper are useful as food additives to increase shelf life of foods because of its medicinal properties [9]. In present investigation, black pepper and turmeric edible coating resulted synergistic increase in both total phenolic content and anti-oxidative potential of Jaggery, and hence the combination of nutritional and medicinal benefits determines them as a functional food.
Microbial Characterization
The total viable count (TVC) in cfu/g (Colony Forming Units per gram) in NC, TPC, TC, PC Jaggery after six months of storage were 5.1x103, 2.98x103, 2.3 x103 and 3.3x103, respectively. Coating of Jaggery samples with edible coating significantly (p≤0.01) affected microbial counts as shown by marked difference in TVC obtained for uncoated and coated samples. The results suggest that coatings of Jaggery samples with edible coating may reduce the microbial deterioration of Jaggery to some extent. Similar findings were reported previously [29,30]. Priya and Garg [9,31] have reported that coatings with powder of black pepper, turmeric, garlic, cumin enhanced the shelf life of potato, tomato taro roots and bottle guard. They further found that synergistic effect of turmeric and black pepper that significantly reduced the microbial spoilage of food stored in refrigerator.
Antibacterial Activity
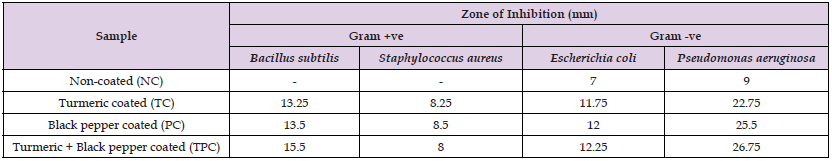
The antibacterial activity of non-coated and coated Jaggery was determined by measuring the diameter of inhibition zone as shown in Table 3 and Figure 1. Among the coated Jaggery, only TPC and PC were effective in inhibiting the growth of gram-positive bacteria compared to NC Jaggery. Against gram-negative bacteria, TPC, PC and TC Jaggery significantly inhibited growth compared to NC Jaggery. Based on the diameter of inhibition zone, it was observed that gram-positive bacteria were more sensitive to the coated Jaggery samples than gram-negative bacteria. Polyphenols and antioxidant properties of the Jaggery may be responsible for the antibacterial activity [30]. In earlier studies, curcumin have been reported to inhibit the growth of antibiotic-resistant Pseudomonas aeruginosa, Acinetobacter baumannii, Klebsiella pneumoniae, Firmicutes, Bacillus subtilis, E. coli, Staphylococcus carnosus, and Mycobacterium smegmatis [11,32,33]. Curcumin has been shown to have a modest level of effectiveness against the parasites Leishmania and Plasmodium falciparum [10,34] Antimicrobial properties of aqueous extract of Curcuma longa have been used by Roy and Garg [34] to enhance the refrigerated shelf life of common foods. Black pepper inhibits the growth of various Firmicutes and Bacteriodetes and was proven to be beneficial in enhancing cell morphology, capsule processing, and lowering urease activity [35]. Black pepper showed anti-bacterial, anti-fungal properties, as well as inhibiting food borne pathogens such as yeast, aflatoxins, and mycotoxin [9]. The present study also depicted that antibacterial activity is proportional to the concentration of phenolic compounds and flavonoids and thus, to the antioxidant performance of the coated Jaggery.
Table 3: Zone of inhibition (mm) of coated and non-coated Jaggery against selective bacterial strains (values are shown in mean of 2 replicates each).
The results of the present investigation suggested that turmeric-black pepper and black pepper coated enhanced the shelf-life with preserved quality of Jaggery for 6 months during storage as it reduced the microbial deterioration of Jaggery and kept the Jaggery in good quality equivalent to fresh. Jaggery is the major cash crop of Indian farmers and they have to sell the product at lower price in the season of its production as the long storage at room temperature spoil the quality of the Jaggery and lowers down its value in the market. The coatings of Jaggery with edible CMC + black + turmeric can enhance its shelf life and provides a solution to the farmers for its long-term storage. Black pepper and turmeric are commonly used in India as herbal products for treatment of various ailments and are part of every Indian kitchen as spice. Further, the coating of Jaggery with these herbal extracts increases phenolic content and antioxidant potential of Jaggery. Hence, turmeric and black pepper coated Jaggery may be used as a substitute for regular Jaggery with additional health benefits.
Conflict of Interests: The authors declare that they have no conflict of interests.


