Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Teshaev BK*, Kholikova AO, Alimova NU and Kenjaeva DI
Received: February 01, 2024; Published: February 13, 2024
*Corresponding author: Teshaev BK, Republican Specialized Scientific and Practical Medical Center of Endocrinology of the Ministry of Health of the Republic of Uzbekistan (RSSPMCE) named a academician Y.Kh. Turakulov, Department of Neuroendocrinology, Medical Administration of the Karshi district of the Kashkadarya region, Kashkadarya branch of the RSSPMCE named after academician. Y.Kh. Turakulov, 100125, Tashkent, st. Mirzo Ulugbek 56, Republic of Uzbekistan
DOI: 10.26717/BJSTR.2024.55.008642
Aim: The purpose of the study is to study the dynamics of height and weight indicators in children and adolescents with type 1 diabetes mellitus during treatment.
Material and Research Methods: On the basis of outpatient observation at the RSSPMCE of endocrinology of the Ministry of Health of the Republic of Uzbekistan named after Acad. YH Turakulov, from 2021 to 2023, 50 patients with type 1 diabetes mellites (DM 1) were examined. At the same time, 23 boys and 27 girls. The average age of boys was 12.7 years, girls 11.8 years.
The patients were divided into 2 groups: group 1 – 30 patients on intensive insulin therapy (IIIT), group 2 – 20 patients on insulin pump injection (IPI). The control group consisted of healthy children, 20 individuals.
Research Methods: General clinical, biochemical, hormonal and instrumental: ECG, ultrasound of the thyroid gland, internal organs, chest x-ray, fundus examination, etc.
Research Results: In patients, there were significant differences in height and weight in girls of both groups compared to the control group (p < 0.05). Moreover, the best height and weight indicators were in group 2 after a year of treatment. Patients in group 1 grew by an average of 7-8 cm, and in the second by 9-10 cm, as well as 6 and 9 kg in weight, respectively. Our study showed that the dynamics of anthropometry data is an effective means of monitoring the effectiveness of treatment for children and adolescents with DM 1, along with laboratory and instrumental studies.
Conclusions: The use of pump insulin therapy has a significantly greater positive effect on all physical development in comparison with intensive insulin therapy. The best results of anthropometry indicators (average height, average weight) were observed in patients of group 2 who received pump insulin therapy.
Abbreviations: Type 1 Diabetes Mellitus; Children and Adolescents; Anthropometry
Type 1 diabetes mellitus (DM -1), an autoimmune disease, is becoming widespread, affecting approximately 490,000 children worldwide. There are various etiological factors that contribute to the spread of its incidence in different geographical locations [1-4]. According to a multicenter study carried out in Germany in 2018, deterioration in metabolic control and dynamics of weight status in adolescent girls indicate eating disorders in the first years after the onset of DM-1 [5]. A total of 31,556 girls aged >6 months and <23 years with DM- 1 from the diabetes cohort were analyzed, including 155 (0.49%) girls with anorexia nervosa, 85 (0.27%) girls with bulimia nervosa, 45 (0 .14%) girls with binge eating disorder and 229 (0.73%) girls with unspecified eating disorders. Patient characteristics, weight changes, number of patients with severe hypoglycemia and diabetic ketoacidosis (DKA), changes in HbA1c levels, pump use. Significant differences in HbA1c levels, prevalence of DKA and severe hypoglycemia between girls with and without eating disorders were found already in first years after the onset of DM- 1. A decrease in body mass index (BMI)-SDS increased the risk of developing comorbid anorexia nervosa (7.1 times [95% CI 3.6–14.3] compared with stable BMI-SDS, 6. 9 times [95% CI 3.4–14.1] compared to the increase in BMI-SDS) [5]. The authors concluded that poor metabolic control, increased incidence of DKA, and severe hypoglycemia in the early years after onset of DM -1 may indicate eating disorders in girls with DM-1, and weight loss is specific to anorexia nervosa. These clinical features should lead to screening for eating disorders, especially in late puberty.
Authors from the USA emphasized that Weight control in DM-1 can be successfully achieved in real clinical practice. Dietary therapy, however, involves reducing energy intake and providing a structured meal plan that is lower in carbohydrates and glycemic index and high in fiber and lean protein. The training plan should include a combination of stretching, as well as aerobic and resistance exercises to maintain muscle mass. Dynamic adjustment of insulin doses is necessary during weight control. Adding anti-obesity medications may be considered. If medical weight loss is not achieved, bariatric surgery may be considered [6]. The authors noted that patients with DM-1 who exhibit clinical features of DM-2, such as obesity and insulin resistance, are considered to have “double diabetes.” Although patients with DM-1 were traditionally thought to have a lower BMI, current research has shown the opposite [5]. The trend towards increasing obesity prevalence is increasing at a faster rate in patients with DM-1 compared to the general population [6]. Currently, about 50% of patients with DM-1are overweight or obese. They also have higher waist and hip circumferences compared to healthy controls [7]. In the Pittsburgh Epidemiologic Study of Diabetes Complications (EDC), which followed adults with DM-1 for an average of 18 years, the prevalence of overweight increased from 29 to 42%, and the prevalence of obesity increased sevenfold from 3 to 23% [8]. Weight gain does not appear to be related to aging but to clinical factors such as insulin therapy [8].
Comorbid diseases, often associated with excess body weight, reduce the benefit of good metabolic control [9]. Thus, weight control in patients with DM-1is necessary due to the well-known relationship between obesity and cardiovascular disease (CVD) [10]. Metabolic disturbances associated with obesity, such as a pro-inflammatory state, are likely to alter the risk of cardiovascular disease in this population [10]. Until now, complications associated with cardiovascular diseases have been the leading cause of death in patients with DM-1 [11]. The above was the reason for our study. In this regard, we have formulated the following goal of research work.
Study the dynamics of height and weight indicators in children and adolescents with DM-1 during treatment.
Based on outpatient observation in Republican Specialized Scientific and Practical Medical Center of Endocrinology of the Ministry of Health of the Republic of Uzbekistan named after academician. Y.H. Turakulov, from 2021 to 2023, 50 patients with DM-1 were examined. At the same time, 23 boys and 27 girls. The average age of boys was 12.7 years, girls 11.8 years. The patients were divided into 2 groups: group 1-30 patients on intensive insulin therapy (IIT), group 2-20 patients on insulin pump injection (PII). The control group consisted of healthy children, 20 individuals.
Inclusion Criteria
Children and adolescents with DM-1.
Exclusion Criteria
Adults, type 2 diabetes mellitus, severe somatic diseases.
General clinical, biochemical (fasting blood glucose, blood glucose 2 hours after a meal, glycemic profile, urea, creatinine, bilirubin, direct, indirect, ALT, AST, PTI, coagulogram, CRP, glycated hemoglobin, etc.), hormonal (TSH, free thyroxine, insulin, C-peptide) and instrumental: ECG, ultrasound of the thyroid gland, internal organs, chest x-ray, fundus examination, etc. Age, sex, height, weight, age at diagnosis, number of insulin injections, total daily insulin dose, number of severe hypoglycemia events (i.e. seizures or loss of consciousness) in the past 3 months, family composition and ethnic status are all filled out by us in a specially designed questionnaire. Statistical software. Microsoft Excel and STATISTICA_6was used for statistical analysis, and p < 0.05 was considered a significant difference. Normally distributed quantitative data were expressed as mean and standard deviation (M ± SD). Analysis and results. Table 1 shows the distribution of patients by gender and age.
As can be seen from Table 1, sick children aged 8 to 12 years were most often observed: 32 cases (64%). Table 2 shows the average indicators of objective examination of boys by group. As can be seen from Table 2, the patients had significant differences in height and weight in boys of both groups compared to the control group (p < 0.05). No significant differences were found in other indicators. Table 3 shows the average indicators of objective examination of girls by group. As can be seen from Table 3, the patients had significant differences in height and weight in girls of both groups compared to the control group (p < 0.05). Table 4 shows the average values of biochemical studies of patients before treatment. Table 5 shows the average values of hormonal studies of patients before treatment. As can be seen from Table 5, patients had significant differences in the content of insulin and C-peptide of both groups in comparison with the control group (p < 0.05). Next, we studied the dynamics of objective examination data one year after treatment in boys and girls of both groups. Table 6 shows the average indicators of objective examination of boys by group.
Note: * - significance of differences compared to control, where * - p <0.05
Note: * - significance of differences compared to control, where * - p <0.05
Table 4: Comparative characteristics of biochemical studies of patients in the study groups before treatment.
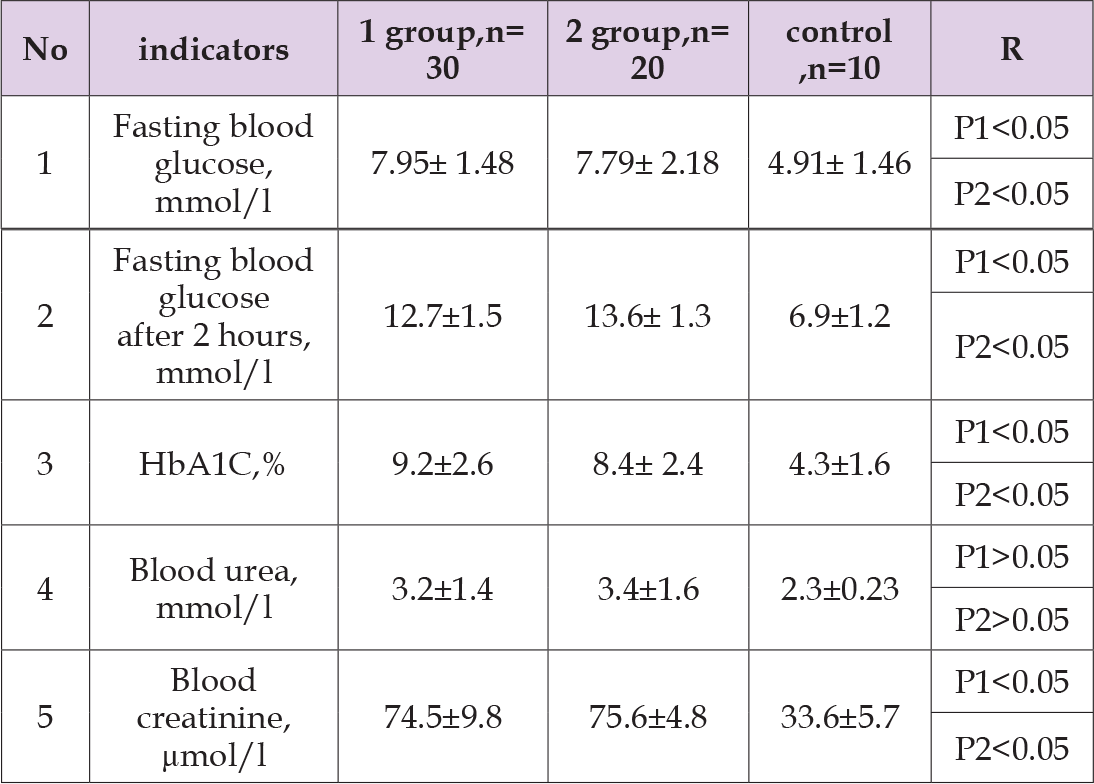
Note: P1 - significance of differences in comparison with control group 1, P 2 - group 2
Table 5: Comparative characteristics of indicators hormonal studies of patients in the study groups before treatment.
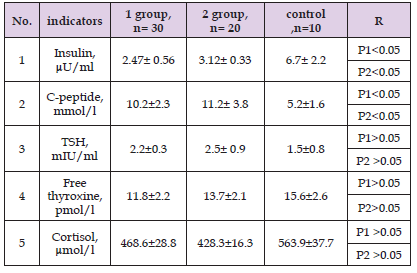
Note: P1 - significance of differences in comparison with control group 1, P 2 - group 2.
Note: * - significance of differences compared to control, where * - p <0.05
As can be seen from Table 6, the patients had significant differences in height and weight in boys of both groups compared to the control group (p < 0.05). No significant differences were found in other indicators. Table 7 shows the average indicators of objective examination of girls by group. As can be seen from Table 7, the patients had significant differences in height and weight in girls of both groups compared to the control group (p < 0.05). Moreover, the best height and weight indicators were in group 2 after a year of treatment. Here, patients in group 1 grew by an average of 7-8 cm, and in the second by 9-10 cm. Next, we studied the dynamics of biochemical and hormonal data one year after treatment (Tables 8 & 9). In terms of biochemical blood parameters, there was a tendency to reduce the average values of fasting blood glucose and glycated hemoglobin and 2 hours after a meal in comparison with the control level, although these values decreased significantly in group 1. At the same time, in group 2, the achievement of the target values of these indicators was reliable both in relation to the control group and with the data before treatment.
Note: * - significance of differences compared to control, where * - p <0.05.
Table 8: Comparative characteristics of biochemical studies of patients in the study groups1 year after treatment
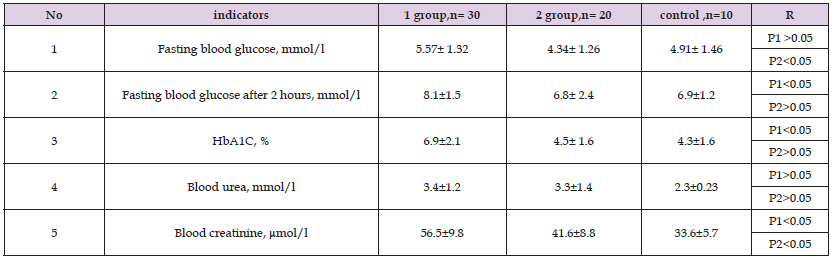
Note: P1 - significance of differences in comparison with control group 1, P 2 - group 2.
Table 9 shows the average values of hormonal studies of patients before treatment. As can be seen from Table 9, in patients there were no significant differences in the content of insulin and C-peptide of both groups in comparison with the control group (p < 0.05). We see that in group 2 patients, the target levels of glycemia on an empty stomach, 2 hours after meals, the level of HbA1C, and insulin were significantly closer to normal than in group 1 (P<0.05). Our study showed that the dynamics of anthropometric data is an effective means of monitoring the effectiveness of treatment for children and adolescents with type 1 diabetes, along with laboratory and instrumental studies.
Table 9: Comparative characteristics of indicators hormonal studies of patients in the study groups after 1 year of treatment.
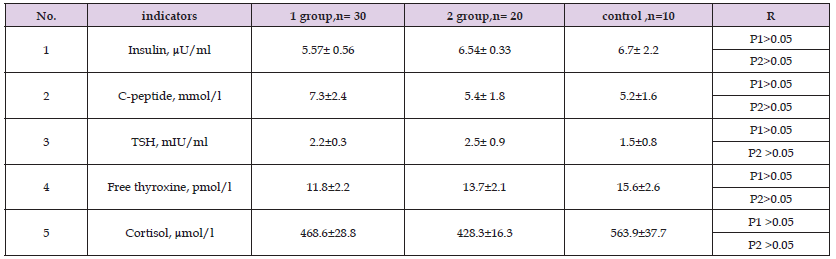
Note: P1 - significance of differences in comparison with control group 1, P 2 - group 2.
1. The use of insulin pump therapy has a significantly greater positive effect on all physical development compared to intensive insulin therapy.
2. The best results of anthropometry indicators (average height, average weight) were observed in patients of group 2 who received pump insulin therapy.


