Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Shakeel Ahmed1, Zakir Hossain1*, Zahirul Islam1,2*, Shubarna Rahman1, Kuldeep Sharma1, Muhammad Fakharruddin Suman1 and Hasan Rabbi1
Received: January 15, 2024; Published: February 12, 2024
*Corresponding author: Zakir Hossain and Zahirul Islam, Bangladesh Institute of Tropical and Infectious Diseases (BITID), Fouzderhat, Chattogram, Bangladesh
DOI: 10.26717/BJSTR.2024.55.008636
Introduction: The RT-PCR is the gold standard for diagnosing COVID-19 infection. However, this method
is time-consuming and requires sophisticated laboratory equipment. To overcome these limitations, rapid
diagnostic tests (RDTs) have been developed to provide a quick and accurate diagnosis of COVID-19 infection.
In this study, we evaluated the performance of an RDT kit Standard Q COVID-19 Ag assay (SD Biosensor®,
Republic of Korea) in comparison with a real-time RT-PCR assay.
Methods: This study was conducted at the Bangladesh Institute of Tropical and Infectious Diseases (BITID)
from July 2021 to June 2022. Patients who presented with symptoms of COVID-19 were included in this
study and compared the rapid Standard Q COVID-19 Ag assay with RT-PCR for the detection of SARS-CoV-2 in
respiratory specimens.
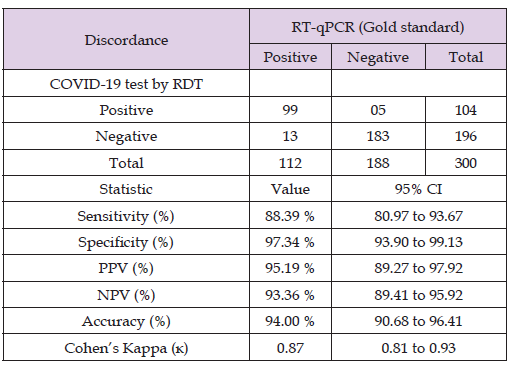
Results: The study included 300 patients, among them 112 tested positives for SARS-CoV-2 through RT-PCR,
of which 99 were also detected as positive by the RDT test. The overall sensitivity and specificity of the RDT
were 88.39% and 97.34% respectively. Most positive cases detected through RT-PCR were identified within
the initial five days of symptom onset for the same samples of RDT. The PPV and NPV of the RDT were 95.19%
and 93.36% respectively. The RDT had 94.00% diagnosis accuracy. The Cohen’s kappa value was 0.87 showing
excellent agreement between the two assays.
Conclusion: Our study shows that the RDT assay has high specificity and accuracy for diagnosing COVID-19
infection. Therefore, rapid diagnostic test can be used as an initial screening tool in high-risk populations,
where rapid detection is crucial, followed by confirmatory testing with RT-PCR assay.
Keywords: COVID-19; SARS-CoV-2; Rapid Diagnostic Test; RT-PCR
Abbreviations: RDT: Rapid Diagnostic Test; PPV: Positive Predictive Value; NPV: Negative Predictive Value; POC: Point of Care; FDA: Food and Drug Administration; IRB: Institutional Review Board; CT: Cycle Threshold
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified in December 2019 in Wuhan, China [1]. The first case of COVID-19 in Bangladesh was confirmed on 8th March 2020 by the Institute of Epidemiology, Disease Control, and Research (IEDCR) [2]. The gold standard for detecting SARS-CoV-2 is real-time reverse transcriptase-polymerase chain reaction (RT-PCR) [3]. To detect viral RNA quantitatively from clinical specimens this molecular technique is very accurate and sensitive [4]. RT-PCR is used by all laboratories in Bangladesh that provide COVID-19 emergency testing. It is a time-consuming and costly procedure, requiring specialized laboratory personnel, advanced equipment, and special laboratory environments. There is an urgent need to adopt more cost-effective Point-of-Care (POC) rapid diagnostic tests (RDTs) for early detection and isolation of infected persons to control transmission of SARS-CoV-2 infection[5,6]. The SARS-CoV-2 RDT was permitted by the US Food and Drug Administration (FDA) in May 2020 to improve containment measures globally [7]. Subsequently, numerous COVID-19 antigen-based RDTs were introduced into the test platform [8,9]. RDT has the benefit of giving interpretable results without special equipment in 15 to 30 minutes. Thus, it has the potential to improve total turnaround time and patient care while reducing the workload of diagnostic hospitals and laboratories [10].
To validate RDT and ensure its widespread use, an evaluation of its performance in various scenarios is required [11]. This validation process helps establish the accuracy and usefulness of RDTs in different settings, ensuring their integrity in diagnosing SARS-CoV-2 infections. In this study, we evaluated the clinical performance of Standard Q COVID-19 Ag kit (SD Biosensor®, Republic of Korea), a rapid SARS-CoV-2 antigen detection assay. The diagnostic performance of this RDT kit was compared to Sansure Biotech’s SARS-CoV-2 RT-PCR detection assay.
Study Design
It was a cross-sectional study where two nasopharyngeal swab specimens were collected from 300 suspected COVID-19 patients presented at the flu corner of the Bangladesh Institute of Tropical and Infectious Diseases (BITID), from July 2021 to June 2022.
Inclusion/Exclusion Criteria
As per the National Guidelines on Clinical Management of COVID-19, we included an individual suspected of having COVID-19 is characterized by an acute onset of fever and cough, or any three or more of the following symptoms: headache, myalgia, sore throat, loss of taste, loss of smell, anorexia/nausea/vomiting, diarrhea, and dyspnea [12]. Patients without symptoms (Asymptomatic) were not included in this study. The patient’s informed written consent was obtained on the day of the sampling. Ethical permission was approved by the Institutional Review Board (IRB) of the BITID.
Specimen Collection
Two Nasopharyngeal swab samples were collected one in a 2mL sample storage buffer (Sansure Biotech, Changsha, China) for SARSCoV- 2 RT-PCR and another one in a 2mL sample storage buffer for RDT at the same time. RDT was carried out immediately after sample collection. Collected specimens in a 2mL storage buffer to be tested for RT-PCR can be immediately processed; specimens to be tested within 24 hours can be stored at 40C. All suspected specimens were treated in a biosafety cabinet with full personal protective equipment.
SARS-CoV-2 Viral RNA Extraction & Detection Via Real‑Time RT‑PCR
Extracting total RNA from 20 μl of nasopharyngeal swab samples, a sample-release reagent (Sansure Biotech, Changsha, China) was used. The extraction of RNA was carried out as per the manufacturer’s instructions. Novel Coronavirus (2019-nCoV) nucleic acid diagnostic kit (PCR-Fluorescence Probing from Sansure Biotech, China) was used for qualitative detection of the N and ORF-1ab genes of SARSCoV- 2 RNA. The manufacturer’s instructions were followed for conducting the reaction, amplification conditions, and interpretation of the results. CFX96Touch™ Real-time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used for amplification. A sample with a Cycle Threshold (CT) value of ≤ 40 for any of the targets (ORF-1ab and N) was considered as a positive case.
Rapid SARS‑CoV‑2 Antigen Detection Assay
Standard Q COVID-19 Ag assay (SD Biosensor®, Chuncheongbuk- do, Republic of Korea) is an RDT test for the detection of SARSCoV- 2 nucleocapsid (N) antigen in respiratory samples. The STANDARD Q COVID-19 Ag Test consists of a nitrocellulose membrane surface with two pre-coated lines: a control line labeled “C” and a test line labeled “T.” Neither line is visible before the application of specimens. The test line area is coated with mouse monoclonal anti- SARS-CoV-2 antibody, while the control line area is coated with mouse monoclonal anti-Chicken IgY antibody. In the SARS-CoV-2 antigen test, a mouse monoclonal anti-SARS-CoV-2 antibody conjugated with color particles serves as the detector. The SARS-CoV-2 antigen in the specimen interacts with the monoclonal anti-SARS-CoV- 2 antibody conjugated with color particles, forming an antigen-antibody color particle complex. This complex migrates through the membrane via capillary action until it reaches the test line, where it is captured by the mouse monoclonal anti-SARS-CoV-2 antibody. In this study, the RDT kit was employed to detect the SARS-CoV-2 antigen in respiratory samples. A volume of 350 μL from the nasopharyngeal swab specimen was introduced into the extraction buffer supplied with the kit. The filter nozzle cap was firmly attached to the extraction tube. Later, three drops of the extracted sample were introduced onto a test device, and the test outcome was interpreted within a timeframe of 15–30 minutes. Samples were treated in a biosafety cabinet with full personal protective equipment. For positive COVID-19 antigen results, two colored lines of control (C) and test (T) lines were presented. The test line was colorless in the absence of SARS-CoV-2 antigen, but the control line displayed a line.
Statistical Analysis
SPSS (Statistical Package for Social Science) for Windows version 23 software was used for the analyses. For each test, sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV), and accuracy were calculated, and 95% confidence intervals were estimated. Agreement between RDT and RT-PCR tests was evaluated using Cohen’s κ value. Poor agreement is shown by a value of k ≤ 0.40, average or good agreement is shown by 0.40< k <0.75, and excellent agreement is shown by k ≥ 0.75 [13]. The individual RT-PCR test result was considered the gold standard for analytical comparison.
In this study, 300 individuals suspected of having COVID-19 were enrolled. Out of the 300 specimens, 112 tested positive for real-time RT-PCR SARS-CoV-2, while 188 tested negative. Of the 112 RT-PCR positive cases, 99 were also positive for the SARS-CoV-2 RDT test, with 13 being falsely negative. Among the 188 specimens that tested SARS-CoV-2 RNA negative, 183 had negative in the SARS-CoV-2 RDT test, with 5 being falsely positive (Table 1). There were 18 discordant results between the two assays. The RDT had an overall sensitivity and specificity of 88.39% (95% CI, 80.97 to 93.67) and 97.34% (95% CI, 93.90 to 99.13), respectively. The positive predictive value (PPV) and negative predictive value (NPV) of the RDT were 95.19% (95%CI, 89.27 to 97.92) and 93.36% (95% CI, 89.41 to 95.92), respectively. The RDT had a diagnosis accuracy of 94.00% (95% CI, 90.68%– 96.41%), and the Cohen’s kappa value was 0.87, indicating excellent agreement between the two assays (Table 1). Among the enrolled patients, 69 (23%) males and 35 (11.7%) females were found positive by the STANDARD Q COVID-19 Ag test, while 79 (26.3%) males and 33 (11%) females were found positive by RT-PCR (Table 2). Patients aged > 18 had 96 (32%) positive cases with RDT and 107 (35.7%) with RT-PCR. Patients aged <18 had 8 (2.7%) positive cases with RDT and 5 (1.7%) with RT-PCR (Table 2).
Table 1: Diagnostic performance of STANDARD Q COVID-19 Ag test kit in detecting COVID-19 with discordance considered (gold standard RT-qPCR).
Out of the 99 RDT positive samples, we observed that n = 40/40 samples had a Ct value of less than 25, followed by n = 47/54 samples with a Ct value between 25 to 30, and n = 12/18 samples with a Ct value greater than or equal to 30 (Table 2). Therefore, the SARS-CoV-2 Rapid Antigen Test had a sensitivity of 100% for samples with a high viral load (Ct<25). The sensitivity was estimated to be greater than 87% and 67%, respectively, for specimens with a medium (25 ≦Ct <30) and low (Ct ≥ 30) viral load (Figure 1). The highest performance of RDT in detecting SARS-CoV-2 was observed up to the fifth day following the onset of symptoms (Figure 2). Additionally, most positive cases detected through RT-qPCR were identified within the initial five days of symptom onset for the same RDT samples (Figure 3).
The gold standard for detecting SARS-CoV-2 is real-time RT-PCR.3 However, this method is time-consuming and requires sophisticated laboratory equipment [5,6]. The STANDARD Q COVID-19 Ag Test is a Rapid Diagnostic Test (RDT) designed to detect the presence of SARSCoV- 2 antigen in nasopharyngeal swab specimens. It is often cost-effective, user-friendly, and can generate results within 15–30 min [14]. In this study, we evaluated the diagnostic performance of the STANDARD Q COVID-19 Ag Test with routine RT-PCR assay to detect SARSCoV- 2 in respiratory samples collected from COVID-19 suspected patients at BITID. In our findings, the overall sensitivity and specificity of the RDT were 88.39% (95% CI, 80.97 to 93.67) and 97.34% (95% CI, 93.90 to 99.13) respectively. The findings of this study were in line with the previously reported study of SARS-CoV-2 antigen-based RDT [15]. The positive predictive value (PPV) of the RDT was 95.19% and the negative predictive value (NPV) was 93.36%. The RDT had an accuracy of 94% among the patients. High diagnostic value is recognized for tests with diagnostic accuracy greater than 90% [16]. The Ct value range indicates that the SARS-CoV-2 gene had a higher viral load during the initial stages of infection, and demonstrated excellent sensitivity. (100%, Ct<25 and 87%, 25≦Ct ≦30). The results were consistent with earlier reports on SARS-CoV-2 antigen-based RDTs [17,18]. Moreover, the assay exhibits low sensitivity (60%, Ct≥30) when detecting samples with a minimal viral load, resulting in a false negative outcome. Patients in the late stages of the infection, which are often accompanied by a low viral load, would not be detected SARS-CoV-2 gene by RDT.
Hence, it is advisable to include an extra RT-PCR test as a confirmatory diagnosis for patients who test negative for antigens [19,20]. It appears that the STANDARD Q COVID-19 Ag test shows potential as a diagnostic tool to substitute the RT-PCR, particularly during outbreaks when there is a rising trend of COVID-19 cases in the population. Such a replacement could expedite clinical decision-making for most suspected patients, aligning with the strategy to halt the current spread of infection in the community [16]. It is noteworthy that the RDT test revealed better diagnostic efficacy when employed in the initial stages of the illness (ideally within the first five days after the onset of symptoms). The findings are synonymous with the Government of Bangladesh’s guidelines [18]. However, this lateral flow immunoassay’s sensitivity and specificity for detecting the SARS-CoV-2 gene are inferior to the Nucleic Acid Test (NAT), which is still considered the gold standard diagnostic test for COVID-19. The study possessed certain limitations such as a limited sample size, a single-center study design, and exclusion of asymptomatic cases. Hence, additional investigations with larger sample sizes, multicenter designs, and the inclusion of asymptomatic cases are necessary to validate the study’s results.
The COVID-19 Ag test demonstrates optimal performance during the first five days of illness when the diagnostic accuracy of this test reached at 94% and the Kappa value showed an excellent agreement (0.87) with the RT-PCR test. We assume there is a potential use of this rapid and simple SARS-CoV-2 antigen detection test as a screening assay, particularly in a region with a high prevalence and limited resource settings. However, due to the chance of false negative COVID-19 Ag test results, we also recommend that the choice of additional testing be made based on the patient’s clinical presentation and, if conceivable, completed by RT-PCR testing.
Md. Zakir Hossain was the principal investigator responsible for study design, laboratory research methodology, investigation, validation and formal data analysis. Md. Shakeel Ahmed was responsible for the project administration and supervision in the study. Shubarna Rahman role was COVID-19 case selection. Md. Zahirul Islam role was laboratory research methodology, investigation, validation and formal data analysis and was responsible for the original draft writing, review and editing of the manuscript. Kuldeep Sharma, Muhammad Fakharruddin Suman and Hasan Rabbi assisted in patient specimen collection and laboratory methodology.
The authors declare that they have no known competing interests or personal relationships that could have appeared to influence the work reported in this paper.
The Rodolphe Merieux Laboratory, Fouzderhat, Chittagong, provided laboratory space, reagents and technical assistance for the duration of the work.


