Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Alieva DA, Mavlonov U Kh, Saydakhmedova MU, Khudaybergenov Sh A, Boboev KT and Urmanova Yu M*
Received: January 31, 2024; Published: February 08, 2024
*Corresponding author: Urmanova Yu M, Republican Specialized Scientific and Practical Medical Center of Endocrinology of the Ministry of Health of the Republic of Uzbekistan named after academician. Y.H. Turakulova, Department of Neuroendocrinology, Tashkent Pediatric Medical Institute, Department of Endocrinology, Pediatric Endocrinology, Department of Surgical Diseases, Bukhara Regional Endocrinological Dispensary, Republican Specialized Scientific and Practical Medical Center for Hematology, Republic of Uzbekistan
DOI: 10.26717/BJSTR.2024.54.008624
Keywords: Boys; Girls; Delayed Puberty and Growth; Gene Polymorphismgnrh1
Gonadotropin releasing hormone (GnRH) is the main hormone of the reproductive endocrine system. The existence of central hormones regulating reproduction was postulated a century ago [1]. In 1910, Crowe et al. [2]. demonstrated that disruption of the hypothalamic-pituitary connection in dogs prevents the onset of puberty. Subsequent studies led to the hypothesis that the pituitary gland is controlled by the hypothalamic factor [3-6]. However, it was not until 1971 that the amino acid sequence of GnRH was determined after extraction from the hypothalamus of thousands of pigs and sheep by the groups of Chally and Guillemin [7,8].
GnRH-secreting neurons are known to arise in the olfactory bulb and migrate to the hypothalamus [1]. Once in the hypothalamus, these GnRH neurons project axons to the median eminence and synchronize GnRH secretion in a pulsatile manner. GnRH is then transported by the portal circulation to the pituitary gland and stimulates the gonadotropins of the anterior pituitary gland, which secrete gonadotropins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH). These gonadotropins then induce steroidogenesis and gametogenesis in the gonads. In addition to extensive physiological research on the central role of GnRH in reproduction [1], human genetic studies have highlighted the critical role of GnRH in the regulation of reproduction [9,10]. Idiopathic hypogonadotropic hypogonadism (IHH) is characterized by the absence of spontaneous sexual development against the background of low levels of sex steroids and gonadotropins with normal pituitary function. When combined with anosmia, this hypogonadotropism is called Kallmann syndrome (KS), while isolated hypogonadotropic hypogonadism with normal sense of smell is called normosmic IHH (nIHH). Studies of patients with nIGG and KS have led to the identification of several genes regulating reproduction. Mutations in KAL1 [11,12], FGFR1 [13], FGF8 [14], PROK2 [15], PROKR2 and CHD7 [16] are thought to impair the development and migration of GnRH neurons, leading to KS. and/or nIGG.
Patients with mutations in PCSK1, which encodes prohormone convertase 1/3, exhibit hypogonadotropic hypogonadism due to abnormal processing of the GnRH decapeptide from its prohormone precursor [17]. Mutations in GPR54 cause nIHH by disrupting normal GnRH secretion [18,19], and mutations in GNRHR, which encodes the GnRH receptor, lead to an inability to respond to GnRH [20]. Mutations in the TAC3 and TACR3 genes, which encode neurokinin B and its receptor, respectively, have recently been implicated in nIHH [21], although their precise functions in reproduction remain unclear. The big omission from the list of genes involved in IHH is GNRH1 itself, which encodes a preprohormone that is ultimately processed to produce GnRH. The results obtained in mice strongly suggest that GNRH1 mutations in humans may cause nIHH. The hpg mouse carries a deletion of Gnrh1, which arose spontaneously and results in a complete absence of GnRH synthesis [22,23]. Male and female hpg mice are sexually infantile, infertile, and have low levels of sex steroids and gonadotropins [22]. In one of the earliest demonstrations of successful gene therapy, reproductive deficiency in hpg mice was reversed using the Gnrh1 transgene [24]. Apart from reproductive phenotypes, hpg mice appear completely normal, although dental abnormalities have recently been reported [25]. The clear association of loss of Gnrh1 function in mice with hypogonadotropic hypogonadism makes the absence of GNRH1 mutations in humans as a cause of nIHH even more puzzling.
GNRH1 is an obvious candidate gene for nIHH, so why have no mutations in GNRH1 been identified yet? [26]. One possibility is that functional mutations in genes encoding ligands occur less frequently than in genes encoding receptors due to differences in the sizes of ligands and their cognate receptors. Encoding a peptide product of only 92 amino acids, GNRH1 represents a smaller "target" for mutations than the 328 amino acids encoded by GNRHR. Indeed, for other ligand-receptor pairs involved in nIHH/KS, fewer mutations have been reported in the genes encoding the ligands (FGF8 and PROK2) than in the genes encoding the receptors [9,10]. An alternative explanation for the rarity of GNRH1 mutations is that they are rapidly eliminated from the population. This may occur due to a failure to pass on mutations to future generations, as would be expected from mutations that cause decreased fertility. Idiopathic hypogonadotropic hypogonadism (IHH) is a condition characterized by the absence of puberty due to low levels of sex steroids and gonadotropins. IHH occurs due to abnormal secretion or action of the main reproductive hormone gonadotropin releasing hormone (GnRH). Several genes have been found to be mutated in patients with IHH, but to date no mutations have been identified in the most obvious candidate gene, GNRH1 itself, which encodes a preprohormone that is ultimately processed to form GnRH. All of the above emphasizes the relevance of this study and was the reason for it.To this end, we screened the DNA of 90 normosmic IHH (nIHH) patients and 20 healthy control subjects for GNRH1 sequence alterations.
Study the meaning of polymorphism GNRH 1 gene (rs 6185, rs1812594) in the development of normosmic IHH in boys and girls.
To achieve this goal, a genetic study was conducted in 90 adolescents diagnosed with iHH, selected during screening in pilot regions of the Republic of Uzbekistan as part of an applied project in the period June-August 2023: Kashkadarya, Jizzakh, Surkhandarya, Namangan regions and the Republic of Karakalpakstan. Among the 90 individuals, there were 73 boys and 17 girls, with an average age of 14.3 years. Disease diagnoses were made in accordance with the latest clinical guidelines. The diagnosis of nIHH was based on the absence of spontaneous puberty and low levels of sex steroids (testosterone <3.4 nmol/L in boys; estradiol, <73 pmol/L in girls) against the background of normal or unreliably low levels of gonadotropins and intact sense of smell.
Patients were Divided into 2 Groups
• Group 1 – patients with nIH + grade 1-2 diffuse goiter with hypothyroidism – 42 patients.
• Group 2 – patients with nIHH – 48 patients.
The control group consisted of 20 healthy individuals of the corresponding average age (10 m boys and 10 girls). All 90 patients underwent a range of studies, including the study of endocrine status, general clinical, biochemical, hormonal (STH, LH, FSH, prolactin, TSH, testosterone, cortisol, free thyroxine, etc.) - in the laboratory of hormonal studies of the Republican Scientific Research and Medical Center of Endocrinology of the Ministry of Health of the Republic of Uzbekistan. In addition, they carried out X-ray (x-ray of the hand and sella turcica, CT/MRI of the sella turcica and adrenal glands in all patients, ultrasound of the genital organs), anthropometric studies (height, weight, height and weight deficit, target height, centile, growth velocity, SDS of height and weight, etc. .) based on the Tanner-Whitehouse international height-weight chart, assessment of the stage of sexual development according to Tanner, karyotyping and other studies. All genetic studies were performed NDC Immunogen test at the Institute of Human Immunology and Genomics of the Academy of Sciences of the Republic of Uzbekistan on the basis of cooperation agreement No. 10 dated December 16, 2021 with the RSNPME Ministry of Health of the Republic of Uzbekistan on the topic “Study of the role of genetic markers PRPP-1, HS6St1, LEP, GNRH-1, Kal-1 in children with endocrine diseases". The material for the study was venous blood samples collected in vacuum tubes with EDTA as an anticoagulant. DNA extraction from peripheral blood was carried out using a commercial reagent kit “Ampli Prime RIBO-prep” (Interlabservice LLC, Russia), according to the manufacturer’s instructions.
Testingpolymorphism GNRH1 rs 6185, rs1812594 in the format Real-Time on a Rotor-Gene Q device (Quagen, Germany) using a commercial test kit from Synthol LLC (Russia) in accordance with the manufacturer’s instructions. Statistical processing of the results was performed using the standard OpenEpi V.9.2 application package.The distribution of alleles and genotypes corresponded to the Hardy-Weinberg distribution law (HW). The odds ratio (OR) was calculated to describe the relative risk of developing the disease. OR>1 was considered as a positive association (predisposition) of an allele or genotype with a disease, and OR<1 (p<0.05) as a negative association.
Table 1 shows the distribution of the selected 90 patients by age and stage of puberty. As can be seen from Table 1, the most common patients among the examined patients were aged 14.7 years - 22 boys and 8 girls (IV stage according to Tanner by age). In this case, the stage of puberty upon examination corresponded to II both 22 boys (30.1%) and 8 girls (47%). In general, when assessing the stage of puberty, it was revealed that 30 adolescents had delayed puberty, that is, puberty corresponded to stage 2 at the age of 13-14 years in both boys and girls. Next, we calculated average anthropometric indicators (Table 2). As can be seen from Table 2, the average height and weight in both groups differed significantly from the data in the control group (p < 0.05). At the same time, in group 1 of patients with nIH+ hypothyroidism, the lowest average values of height and weight were observed in comparison with group 1 of patients with nIHH. The next step in our research was to study hormonal disorders (Table 3). As follows from Table 3, the studied patients had a significant decrease in basal values of LH, FSH (p < 0.05) compared to the control group, as well as significantly low levels of free testosterone (fT) in blood plasma (p < 0.05) at background normoprolactinemia. Thus, the patients were diagnosed with hypogonadotropic hypogonadism.
Table 1: Distribution of selected 90 patients by age, gender and 5 stages of puberty according to J Tanner.
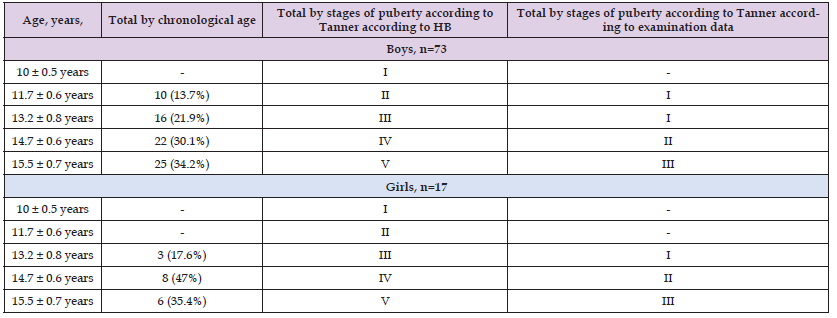
Note: Numerator - boys, denominator - girls, * - significance of differences compared to control, where * is p < 0.05; BA – bone age, ChA- chronological age.
Note: P - significance of differences compared to the control group (P <0.05). The table for comparison shows fluctuations in hormone levels from 11 to 16 years of age in the control group (healthy individuals).
Table 4 gives average hormone values in the studied girls on the 14th day of the cycle.As follows from Table 4, on the 14th day of the cycle, the studied patients had a significant decrease in basal values of LH, FSH (p < 0.05) compared to the control group, as well as significantly low levels of E2 in the blood plasma (p < 0.05) against the background normoprolactinemia. Thus, the patients were diagnosed with hypogonadotropic hypogonadism. Next, we performed calculations for genetic studies (Table 5). The control group consisted of 20 healthy children of the corresponding age (10 boys and 10 girls) with the T/T genotype. Among the examined individuals, the T/T genotype was detected in 64 (71%) patients, the T/C genotype in 22x (24.4%) and 4 (4.4%) had a mutation of the C/C genotype. It should be noted that in 5 (6.6%) cases the rs 6185 polymorphism of the GNRH 1 gene was found.Polymorphism rs1812594 of the GNRH 1 gene was observed in 4 patients (4.4%). According to the literature, the reported allele frequency of this SNP is 18–30% in Caucasians and 52–61% in Asians.One patient and one control subject were found to be heterozygous for SNP rs6186, which has an allele frequency of 1-4% [26].
Note: p - significance of differences compared to the control group (p <0.05). The table for comparison shows fluctuations in hormone levels from 11 to 16 years of age in the control group (healthy individuals); E2 – Estradiol.
Table 5: Clinical data of 10 patients examined with analysispolymorphismGNRH 1 gene (rs 6185, rs1812594).

1. The most common patients among those examined were aged 14.7 years - 22 boys and 8 girls (IV stage according to Tanner by age). In this case, the stage of puberty upon examination corresponded to II both 22 boys (30.1%) and 8 girls (47%).
2. In general, when assessing the stage of puberty, it was revealed that 30 adolescents had delayed puberty, that is, puberty corresponded to stage 2 at the age of 13-14 years in both boys and girls. At the same time, in group 1 of patients with nIH+ hypothyroidism, the lowest average values of height and weight were observed in comparison with group 1 of patients with nIHH.
3. Our results confirm that gene polymorphism GNRH 1 is the genetic cause of nIHH. At the same time, out of 90 patients with clinical and hormonal data, nIHH was found in 5 (6.6%) cases. polymorphismrs 6185geneGNRH 1and in (4.4%) -rs1812594.


