Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Sahar Abd Al-Daim*
Received: January 02, 2024; Published: January 10, 2024
*Corresponding author: Sahar Abd Al-Daim, Environmental Virology Lab 176, Water Pollution Research Department, Environment and Climate Change Institute, National Research Centre, 12622-Dokki, Cairo, Egypt
DOI: 10.26717/BJSTR.2024.54.008556
Background: It’s no secret that the microbiological safety of our food and the environment we live in is
important issue. With ever-increasing production and a global market driven by demand, there’s a growing
need for high-quality. In recent years, there has been an increased focus on important viral agents related to
food/water contamination in both research and routine diagnostics. Food contaminated by hepatitis A virus
(HAV) is responsible of the 2–7% of all HAV outbreaks worldwide.
Aim: The purpose of the current study was to evaluate fresh vegetables and irrigation water. which in most
cases contaminated with fecal material of both animal and human. RNA of HAV was detected by nested RTPCR
method. One hundred fresh vegetables and twelve irrigation water samples were collected (January-
March 2022).
Result: Our result showed contamination of HAV in 3/12 (25%) in irrigation water and 2positive samples
were detected in vegetables (one sample lettuce collected from Aflaqa village and one lettuce sample collected
from Zawayat Ghazal village) representing 2%.
Conclusion: It may be concluded that fecal contaminated water is unsafe for irrigation because of the health
risk associated with such practices, and continuous monitoring of vegetables and irrigation water is very
important issue to protect human health and lower cost of medication resulted from consuming contaminated
food. This research raises concerns about the presence of human hepatitis A virus in fresh vegetables,
especially when it is consumed raw.
Keywords: Hepatitis a Virus; Enteric Viruses; Health Risk; Vegetables; Irrigation Water; Foodborne
Over 250 causes of foodborne illnesses have been found worldwide, with bacteria, viruses, protozoa, and fungus accounting for the majority of these cases [1]. Foodborne/waterborne virus infections represent a serious risk to public health and have a significant financial impact on both developed and poor nations. Enteric viruses are the primary cause of the majority of foodborne/water borne infections and outbreaks. Egypt is categorized by the World Health Organization as one of the regions having intermediate to high levels of enteric virus endemicity [2-4]. The Egyptian population has a high incidence of many intestinal virus infections, including human rotaviruses, human noroviruses, human astroviruses, human adenovirus, and hepatitis A and E viruses [5]. The Hepatitis A virus (HAV), a member of the Picornaviridae family, single strand RNA with positive polarity. HAV is mainly transferred by the fecal–oral route, unlike the sexually transmitted hepatitis B and C viruses, which cause chronic liver disease (they are a bloodborne infection that is responsible for 96% of all hepatitis deaths). On the other hand, HAV-induced acute liver failure can be fatal. 7134 people worldwide died from HAV in 2016, according to WHO estimates (which accounts for 0~5% of total viral hepatitis deaths [6].
Food contaminated by hepatitis A virus (HAV) is responsible of the 2–7% of all HAV outbreaks worldwide. Outbreaks caused by it occur more frequently in settings such as hospitals, daycare centers, schools, and in association with foods and food service establishments. HAV is ranked sixth out of the top 10 foodborne pathogens and is the fifth most often reported infectious disease in the US [7]. Water samples taken from the Mediterranean Sea in the Alexandria Governorate, northern Egypt, revealed the presence of many enteric viruses [8]. Consuming items contaminated by faces is the main way that the virus is spread [9]. HAV contamination of food can happen in a number of ways, including: fruits and vegetables grown in and/ or irrigated by fecal materials; food processing and preparation on fecal equipment; and handling of ready-to-eat food by contaminated individuals with inadequate personal hygiene [10,11]. Unsatisfactory production procedures, inadequate waste disposal systems, and unhygienic environments in food facilities can all lead to food contamination [12]. If any possible health and environmental hazards are avoided, primarily those related to food safety and the irrigated products, then the reuse of treated wastewater as agricultural water should be encouraged [13]. The aim of present study was to evaluate fresh vegetables and irrigation water from four villages located in Damanhur, Egypt for the searching either vegetables or irrigation water were contaminated with RNA genome of HAV or not as an important step to assist in protect our health and environment.
Sample Collection and Study Area
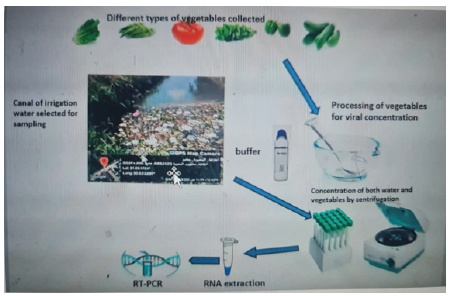
Samples comprised from fresh vegetables and herbs include lettuce (Lactuca sativa), tomatoes (Lycopersicon esculentum), Mint (Mentha), Okra (Abelmoschus esculentus), Pepper (Capsicum annuum), and Cucumber (Cucumis sativus). One hundred collected fresh vegetables and herbs were collected as follow, Tomatoes (n=15), lettuce (n=18), Mint (n=15), Okra (n=18), Pepper (n=18) and Cucumber (n=17) were randomly sampled from various main marketplaces located in four regions of Damanhur city center, Egypt (Aflaqa village, Qaraqis village, Zawayat Ghazal village, and Senhor village). Although 12 irrigation water samples were collected from the canal responsible for irrigation in each four mentioned village (all villages where agriculture fields are irrigated directly with domestic sewage or with the canals contaminated by domestic sewage). Vegetables, herbs and irrigation water samples were collected through time of sampling (January -March 2022). Samples were aseptically collected in nylon bags in case of fresh vegetables and in clean plastic bottles in case of irrigation water samples and transported immediately to the laboratory in a cooler ice box, frozen in -20. Behera Governorate is a coastal governorate in Egypt. Located in the northern part of the country in the Nile Delta, its capital is Damanhur.
Processing of Vegetables Sample for Virus Concentration
Viruses were released from the food surface by gentle shaking with 60 ml of elution buffer (50 mM glycine, 100 mM Tris, 1% [wt./ vol] beef extract pH 9.5) for 30min at room temperature and 30 g portions of each sample were homogenized and ground in a clean mortar and pestle for 15 min at room temperature with part of elution buffer. The elution buffer was collected and then transferred into a Falcon tube and centrifuged at 2000 rpm for 10min to remove particulate debris. The recovered elution buffer was then adjusted to pH 7.0, with 9.5 M HCl and centrifuged at 3,500 g for 15 min, and the supernatant was then transferred to new falcon virus concentration in the following two stages: first, the viruses were absorbed onto 0.45-nm nitrocellulose and eluted by washing the filter three times with 30ml of 3% beef extract, pH 9.0; then, the three washings were pooled and reconcentrated by organic flocculation [14,15]. Then final pellet was dissolved in distilled water and stored -20C in 2 ml cryovial until used in RNA extraction and RT-PCR.
Processing of Irrigation Water Sample for Virus Concentration
Two liter of irrigation water were collected from four selected villages for three months. An adsorption-elution method using nitrocellulose membrane was used to concentrate viruses from irrigation water samples as described previously [16]. Briefly, irrigation samples (2 L) were adjusted to PH 3.5 using 1M HCL (0.5 mol l−1) and the sample was passed through stainless holder under pressure. Although, MgCl2 (2.5 mol l−1) was added to the sample to a final concentration of 0.05 mol/L. After filtration through a nitrocellulose cellulose membrane filter (0.45 μm, ADVANTEC, Tokyo, Japan). The viruses were eluted from the membranes, using 100 ml of a 3 % beef extract glycine solution (pH = 9), followed by organic flocculation precipitation procedure [14]. The eluted solution was stirred for 30 min in magnetic stirrer and then PH was adjusted to 3.5 and centrifuged at 4500 rpm for 20 min, then the supernatant was discarded and the pellet were dissolved in 2ml of 0.05M disodium hydrogen phosphate solution and stored at -20 until being used in RNA extraction. The obtained eluent was treated with antibiotic antimycotic to kill any bacteria or fungus present in the sample.
RNA Extraction of HAV
RNA was extracted from samples according to [17] with minor modification using Guanidine isothiocyanate (BIO BASIC, Germany). Briefly, Nucleic acid was extracted from 100μl of the concentrated samples suspension then 40 μl of silica ‘SiO2’ matrix was added and 900μl of lyses buffer. The mixture was vortexed thoroughly and the tubes were laid sideways for 10 min, and then centrifuged at 14000 rpm for 30 second and the supernatant discarded. Washing buffer (1000μl) were added to the sediment and vortex thoroughly then centrifuged at 14000 rpm for 30 sec. the supernatant were discarded. 1000μl of 70% ethanol is added two times after centrifugation of sample at 14000 rpm for 2 min. the supernatant were discarded. 1000μl of acetone added to the sediment and the sample vortexed well and centrifuged at 14000 rpm for 3 min. The acetone totally removed by pipette and the samples sediment dried at 50-56°C for 10 min. then 75μl of nuclease free water (DEPC) added, and 1μl of RNase inhibitor.
The samples were incubated at 50-56 °C for 15min. and vortex in between and centrifuged at 14000 rpm for 3 min. 65μl of supernatant transferred to a fresh sterile tube and centrifuged at 14000 rpm for 3 min. and 55μl of supernatant transferred to a fresh sterile tube and centrifuged at 14000 rpm for 3 min. and 50μl of supernatant transferred to a fresh sterile tube and centrifuged at 14000 rpm for 3 min. that to totally avoid presence of silica in the extracted RNA. The samples were stored at-20°C. we make pooling for100 fresh vegetables samples to 25 samples (4 samples in each pool) to minimize the cost because there was shortage in fund and the material is very expensive.
cDNA Synthesis and PCR
cDNA was synthesized by mixing 10 μl of the extracted RNA, 1 μl of specific reverse primer, then we add 4 μl of dNTP, 0.5μl of Maxima Reverse Transcriptase (Thermo scientific 200 U/μl), 5μl of 5x RT buffer, 0.5μl RNase inhibitor, 4 μl DEPC-treated water to make 25 μl reaction mixture. The mixture was heated at 25°C for 30 min, 42°C for 30min, followed by 95°C for 5 min, to stop working enzyme and then chilled on ice. The polymerase chain reaction used to detect HAV were consisted of two rounds of PCR in the first round we used 3ul of synthesized cDNA and 12.5ul of Dream Taq PCR master mix ,1ul of forward primer A-F1,1ul of A-R1reverse primer and 7.5ul of DEPEC water to obtain reaction mixture of 25ul with temp condition of 5min at 95C followed by 40 cycles of denaturation 94c for 45sec, annealing 55C for 45 sec and extension 72C for 1min, with final extension at 72C for 10min and the obtained PCR product were under go to second round of PCR condition with another two specific primer with 1ul of PCR product of first round and 12.5ul of dream Taq master mix ,1ul of forward primer A-F2 , 1ul of reverse primer A-R2 and 9.5 ul of DEPEC water with PCR condition as follow : primary denaturation 95C for 5min,35cycles of 94C fo30sec ,62C for 45sec and 72C for 1min with final extension time at72C for 5min. using 1.5% agarose gel (electrophoresis grade, iNtRoN, Cat.No.32033) containing 0.5μg ethidium bromide. The PCR product was run at 100 V for 45 min in submarine electrophoresis (model: HB1214, RATED: 0-150V,0-100MA) (Table 1), and visualized under transilluminator (SPECTROLINE, MODELTM- 312A), electrophoresis power supply (CONSORT,3000v-300Ma, E833) comparing to the low-grade DNA ladder (NORGEN, cat# 11400, Canda) (Figure 1).
According to result, we observed contamination of 3 out of 12 irrigation water samples with HAV RNA representing (25%) as shown in (Table 2) (Figure 2), also we have detected HAV RNA in 2 fresh vegetables samples (Lactuca sativa) 2/18 representing 11.1% in compared with same samples of collected fresh vegetables and represent 2% when compared to total collected vegetables screened in our study (Figure 3).
The interest in viral agents has grown further in recent years as a result of the global pandemic caused by the novel coronavirus. There is also no information available in Egypt about foodborne outbreaks, the number of cases, the causative agent, the point of infection, and the implicated food. Nonetheless, minimally processed fruits and vegetables supplied from Egypt are associated with an increasing number of enteric virus outbreaks [2-4]. As a result, several importing nations have outlawed the import of specific Egyptian commodities [19,20]. From farm to fork, food contamination with HAV can happen at any stage of the food chain. contact with improperly treated sewage or water contaminated by sewage, with the most frequent sources of HAV contamination in food, contaminated surfaces and, to a lesser extent, infected food handlers. It may be concluded that crop irrigated with sewage water may be screened not only for HAV, but also for other pathogenic microorganisms, especially human pathogens of viral, bacterial, fungal and other origin [21,22].
Based upon this study, the human consumption of vegetables grown on fields irrigated with fecal contaminated water is not recommended. so, Monitoring HAV in fresh vegetables as well as irrigation water can, in fact, be a helpful tool in lowering the risk of foodborne illness associated with their presence. This research is first report in screening of HAV in vegetables and irrigation water in Damanhur villages selected. The government and public society must take immediate action against such bad practices. The goal of teaching individuals about this hidden health danger may help mobilize society against such activities, which is widely desired. Globally, there were very few studies on monitoring of vegetables and herbs for the presence of food borne viruses and detection. investigation studies carried out only after outbreaks occurs in the area of origins of certain hospitalization. As examples of this, there have been several outbreaks of severe hepatitis and foodborne gastroenteritis in the US, Canada, Australia, and European Union nations [5]. The intake of minimally processed foods imported from Egypt was linked to some of these outbreaks, suggesting that Egyptian foods may also be somewhat to blame for the high prevalence of enteric virus infections among Egyptians [5]. It is challenging to assess the virological safety of Egyptian foods in the lack of governmental foodborne-pathogen surveillance programs. In other study carried out by Luciana et al (2002) [23] lettuce appears to have the maximum adsorption capacity and the best circumstances for viral persistence.
Because of its large size and wrinkled texture of leaves. Another outbreak, occurred on the first three months of 2002, there was a noticeable rise in the number of hepatitis A cases recorded in Auckland, New Zealand. Reverse transcription polymerase chain reaction (RTPCR) was used to identify HAV in stool samples from many instances. An epidemiological analysis showed a strong correlation between the illness and raw blueberry consumption, which was present in majority in stool samples taken from the patients. Although another significant hepatitis A outbreak in Pennsylvania in November 2003 was connected to eating green onions at a restaurant [24]. Very few research, meanwhile, have examined the simultaneous identification of these viruses in irrigation water and fresh vegetables worldwide not in Egypt only. In the present study we detect presence of HAV RNA in 2 samples of lettuce representing 2% which is very low in compared to study carried in Mansoura and Giza regions, Egypt which reported presence of HAV in 10/48 in fresh produce [25] which may be resulted from different studied region and different molecular method. Another study reported that 9.2% of contaminated lettuce by infectious virus particles comes from contaminated hands [26,27]. In the farm and during the growing stage, food can become contaminated by contact with sewage, contaminated fertilizers, or the use of contaminated irrigation water [28].
One of the major drawbacks of the present study the inability of the RT-PCR method, which was employed to identify viruses, to distinguish between infectious and non-infectious viral particles in the positive samples. Viral particles that are infectious or non-infectious can be detected by molecular tests. According to Hamza et al. (2009), the identification of viral nucleic acid in a sample does not always imply the presence of infectious virus particles in that sample [29]. Thus, fresh produce’s involvement as a vector for the spread of infectious enteric viruses cannot be confirmed by molecular technique- based enteric virus identification. There is a lot of reports and research conducted worldwide in monitoring and detection of HAV in irrigation water, in Egypt, [24] reported detection of HAV in 31.9% which relatively higher our detection rate 25%, We can conclude that more urgent to apprise all those connected with the food industry of the importance of environmental and other control measures based on the most recent scientific data available on HAV. No amount of public information on hepatitis A and the agent that causes it is likely to lessen the risk of the disease spreading through food unless it is made available to everyone involved in the food business in a format that is clear and informative. This is especially crucial for employees who often touch food at different phases of preparation and sale.
Not applicable.
Not applicable.
All data generated or analyzed during this study are included in this published article.
No competing interests.
This research received no external funding.
S. Abd Al-Daim, Conceptualization, methodology, investigation, Writing—original draft preparation, reviewing, sampling.
I would to thank Dr Ahmed M. El-Bakry, Department of Pests and Plant Protection, Agricultural and Biological Research Institute, National Research Centre, for assist me in plant collection and classification.


