Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Khodakov Gennadii Vasilyevich1* and Ustimenko Valery Nikolayevich22
Received: December 09, 2023; Published: January 05, 2024
*Corresponding author: Khodakov Gennadii Vasilyevich, Associate Professor, Department of Technology and Equipment for the Productionof Fats and Essential Oils, V.I. Vernadsky Crimean Federal University, Vernadsky avenue, 4, Simferopol, 295007, Russia
DOI: 10.26717/BJSTR.2024.54.008542
By chromate-mass spectrometer method it was positioned the qualitative and quantitative maintenance of terpene’s components in hexane extracts obtained from plants samples of the species Artemisia balchanorum Krasch, corresponded to all phases of its vegetation. The relative graphic-analytical and template analyses of the received experimental and modelling settlement data based on own methodical approaches are carried out. One determined the kinetic dependences for a dynamical accumulation of monoterpene’s components in investigated plants according to degrees of ring formation to study the influence of a plastid membrane on the kinetic characteristics of the bicyclic monoterpenes formation, and also to obtain the data of the biosynthesis localization of acyclic and monocyclic monoterpenes in a plant cell.
Keywords: Monoterpenes; Kinetics; A Biosynthesis Constant; Plastid Membrane; Artemisia
Abbreviations: DMAPP: Dimethyl-Allyl-Pyrophosphate; IPP: Isopentyl-Pyrophosphate; GPP: Geranyl-Pyrophosphate; FPP: Farnesyl-Pyrophosphate; GGPP: Geranyl-Geranyl-Pyrophosphate
Kinetics of Monoterpenes
A – plastid glucose;
B – isoprene;
R – share part of the plastid glucose;
X – carbocation of dimethyl-allyl;
Y – carbocation of isopentyl;
X2 – normalized mass fractions of the acyclic monoterpenes in the plant;
Y2 – normalized mass fractions of the monocyclic monoterpenes in the plant;
Z – normalized mass fractions of the bicyclic monoterpenes in the plant;
ki, kri: kinetic constants;
k24 – constant of the equlibration;
vX, vY, vZ – velocities of the monoterpene’s hydrocarbons dispersion in the surroundings, accordingly;
-vector’s modules of the normalized productivity;
ti – the dimensionless time by units of the first phase season (I) during the vegetation.
The formation of any terpenes includes the condensation of dimethyl-allyl-pyrophosphate (DMAPP) with isopentyl-pyrophosphate (IPP), catalyzed by a chain of prenyl-transferases, which are specific by its longwise. It is added to DMAPP step by step the IPP where geranyl-pyrophosphate (GPP) is primary formatted. This process is catalyzed by the ferment geranyl-pyrophosphate-synthase, and further there is an escalating of the hydrocarbon chain with the formation of the highest terpenes, and GPP is considered itself as the source of all monoterpenic hydrocarbons multiformity. Geranyl-pyrophosphate-synthase is active as the ho modimerizator for some kinds, for example Abies grandis (Tholl [1,2]) or the heterodimerizator for other kinds, where mint peppery Mentha piperita is the typical example (Burke [3]). The localization of the N-end in structure of geranyl-pyrophosphate-synthase is found in the majority of vegetative kinds cell plastids (Soler, et al. [4,5]). The combination together of one molecule DMAPP and two molecules IPP leads to the formation of farnesyl-pyrophosphate (FPP), which is the first predecessor of the sesquiterpenes majority and which is catalyzed by (FPS). Genomes of plants, apparently, code various isoforms of FPS which are localized in cytosols, plastids, mitochondrias or peroxisomes (Cunillera, et al. [6-9]).
In an tomato the cis-prenil-transferase Z,Z-farnesyl-pyrophosphate-synthase is localized in the plastids where it participates in the biosynthesis of the flying sesquiterpenes (Sallaud, et al. [10]). Diterpenes are formed from geranyl-geranyl-pyrophosphate (GGPP), which is synthesized itself by the catalysis of geranyl-geranyl-pyrophosphate-synthase from one molecule DMAPP and three molecules IPP. This ferment exists in plastids, endoplasmic reticules and mitochondrias (Cheniclet, et al. [11-14]). The biosynthesis, which leads to assemblage of the structural foundation and makes a variability of each terpene’s group is catalyzed by terpene-synthase. This type of ferments is responsible for a synthesis of hundreds structurally various hydrocarbon’s structural foundations. The ring formation principle of diterpenes by the use of two enzyme’s complexes diTPS I and diTPS II is known. Highly active intermediate compounds of carbocation of diterpenes result from the eliminating of the diphosphate substrate group on the ferment diTPS (by the class I), which completes the cascade of ring formations, by the elimination of a carbocation’s charge by means of the deprotonation or the electrophilic attacks by a molecule of water (Gong [15]). At the same time to form a structural variability of monoterpenes it is not required the preliminary multiple increasing of the isoprene unity, but it formally results from the binocular biochemical reaction of two intermediates combination (Kintya [16]) on the corresponded ferments.
We introduce the kinetics of such process as the uniform mechanism of the monoterpenes biosynthesis in the stream of substances for some plants samples by the genus Artemisia (Ustimenko [17]). Terpene-synthase, which ensure such processes, often catalyze the formation of several products from prenyl-diphosphate substrate received in turn by the catalytic way. Prenyl-diphosphate substrate includes highly active carbocations in the form of intermediate products (Degenhardt [18]). As a rule, monoterpene-synthases are localized in the plastids whereas sesquiterpene-synthase exist in the cytosols of cells (Chen [19]). Nevertheless, the localizations of the monoterpene-synthase in the cytosol (Aharoni, et al. [20]) and that of the sesquiterpene-synthase in the plastids (Sallaud, et al. [10]) are known. As far as the plastid methyl-erithritol-phosphate’s biosynthesis way (DXP) it is characterized by the higher intensity in comparison with the biosynthesis by the mevalonate way (MVA), which are localized in the cytosol and peroxisomes, and it is important to notice that through the plastid membrane there is an interchanging of isoprene’s intermediates, and the carrier of initial IPP, DMAPP, and also formed terpenes (Lange [21]) is realized. The work purpose is to determine a monoterpene’s hydrocarbons accumulation dynamics in the sort plants samples of species Artemisia balchanorum Krasch.
Due to find the specific influence of a plastid membrane on the cinetics of biochemical processes and the system changes during the formation of monoterpenes, which have descended in the plants as a result of the directed influence by the person. The scientific novelty consists of that is proposed as the markers for the detection of the person’s influence on vegetative taxons to apply not so the known substances, but also the kinetic constants (or their interrelations), which are included in the biochemical mechanism of such substances formations (the kinetic chemotaxonomy). For this purpose, for the first time the cellular mechanism of the monoterpene’s hydrocarbons biosynthesis is presented with the estimated kinetic data resulted for the plants of species A. balchanorum Krasch.
Above-ground parts of the plants samples by species Artemisia balchanorum Krasch were collected in the Nikitsky Botanical Garden (Crimea) during its four phases of vegetation, namely:
1. A spring regrowth of propagules
2. Budding
3. Mass blooming
4. And ripening of seeds.
Each vegetative object by the mass 100 g was extracted twice by hexane using the infusion without heating during three days. Then aggregated extracts were filtered off and were combined together according to its repeated Ness and were boiled down in a vacuo up to 1/3 volumes. Boiled down extracts of each vegetative phase were investigated with the help of the chromate-mass spectrometer method using the device Agilent Technologies 6890 (USA), and 5973 mass spectrometer and database NIST 02. Chromatography conditions were next: the quartz column (30 m х 0,25 mm); the gas-carrier was helium, the expense of the gas-carrier was 1 ml/minute, the evaporator temperature was 249 оС, the temperature program was from 50 up to 230 оС (3 С/minute), the volume of the introduced sample was 0,1 mkl. The received results of component’s compositions we distributed according to degrees of the ring formation and presented in (Table 1). Research methods were next: the relative grafic-and-analytical and template analyses (Panitchev [22]) of the received experimental and modelling settlement data about the dynamics of the monoterpene components accumulations in the hexane extracts from the plants samples by species Artemisia balchanorum Krasch during all phases of its vegetation. We combined the mass fractions of monoterpene’s components according to the ring formation degrees (acyclic, mono- and dicyclic) and reduced for each of them their relative general quantities, which are named further as normalized mass fractions.
Table 1: Qualitative composition and the quantitative maintenance of monoterpene’s components in three hexane’s extracts from the plants by species Artemisia balchanorum Krasch. during all vegetation (the phases I-IV).
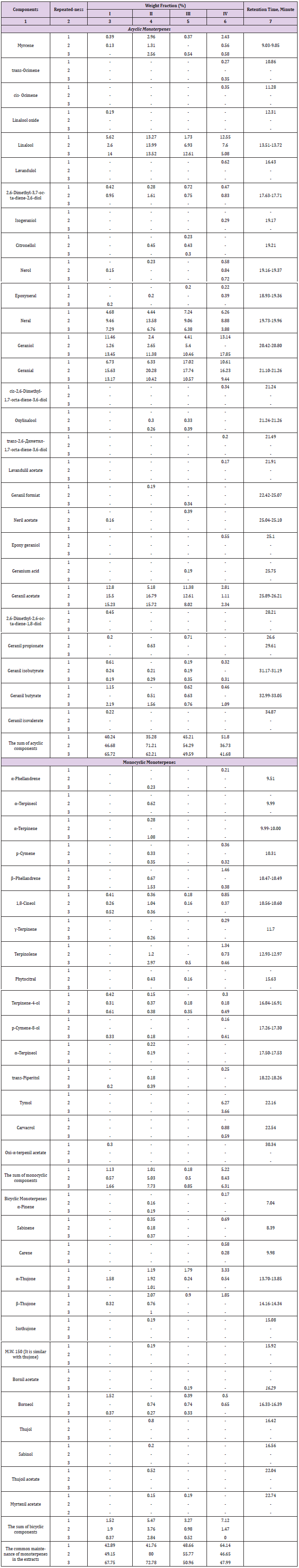
The system of the differential equations, which reflects the changes of normalized mass fractions during the vegetation of the plants, was solved by the numerical Runge-Kutta method of the fourth order (Djaconov [23]) with the help of the programe maintenance in MathCad. One used the Fisher's method (Ustimenko [24]) to verify the adequacy of the created kinetic model to the received experimental data. We presented the normalized mass fractions as the vectors in the tridimensional system of orthogonal co-ordinates, and the vector modules of the normalized mass fractions (efficiency) for monoterpene’s components were calculated by the representation in the template form of the nonlinear kinetic equations decisions. The kinetic equations demonstrated a dynamics for each stages of the connatural monoterpene’s biosynthesis are the next (Ustimenko [17]):
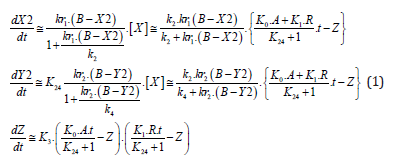
where

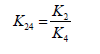
The residual quantity of monoterpenes in the samples of plants according to modelling calculations was defined with the help of the equations (3) using the data (Ustimenko [17]):
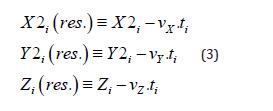
where X2i, Y2i, Zi – normalized concentrations of the monoterpenes as the numerical solutions of the equations system (1); vX, vY, vZ – are the velocities of monoterpene’s hydrocarbons dispersion in the surroundings, accordingly; ti – the dimensionless time in unities of the season first phase (I) in the vegetation. The weight fractions of the residual content of monoterpene’s hydrocarbons types (wXi, wYi, wZi) in the samples of plants (normalized concentrations according to the condition wXi++wYi +wZi=1) as accordintly to the experimental data (in the composition of the extract), as by the modelling calculations (in the plants) were defined by the equations (4) (Ustimenko & Khodakov, 2018 [24]):
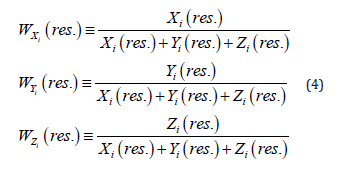
It is expedient to describe the normalized concentrations (weight fractions) as the vectors in tridimensional system of orthogonal co-ordinates. As a result there are possible to calculate the vector modules of normalized weight fractions in plants (efficiency) for monoterpene’s ingredients
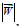
To continue the research of the hydrocarbons biosynthesis kinetics in the absinthes, which grow in nature or are cultivated by the person in Crimea (Khodakov [25,26]), we detailed studied the dynamics of qualitative composition and the quantitative maintenance of monoterpene’s components in the heksane’s extracts from three plants samples of species Artemisia balchanorum Krasch. produced by man-made (Table 1). One made the researches during all vegetation phases, namely:
1. A spring regrowth of propagules,
2. Budding,
3. Mass blooming
4. And maturing of seeds.
The plants samples are the result of the controlled breeding due to obtain the products for perfumery-cosmetic and the food-processing industry. The components of the monoterpene’s nature has been identified in the hexane’s extracts. The identified monoterpene’s components were distributed according to the groups, such as: acyclic, mono- and bicyclic and we named the individual and group maintenance in each of them. The basic terpene’s components in the extracts are six substances: linolool, geranial (b-сitral), neral (a-сitral), geraniol, geranyl-acetate. Their quantity during the period of vegetation is changed, but it remains on enough high level. Monoterpene’s components are presented by the acyclic, bi- and three-cyclic structures, but at the same time the qualitative maintenance of acyclic substances is prevailable (Table 1). The qualitative diversity of the monocyclic and bicyclic monoterpenes is less, than the acyclic monoterpenes. The acyclic monoterpenes are absolutely prevail by the quantitative maintenance. Group dynamics of the monoterpene’s components accumulation is presented in (Table 2). The maintenance of the main components varies during the vegetation. The accumulation dynamics of the main acyclic monoterpene’s component in the plants samples during the vegetation is practically the same in all components, except a geraniol and geranyl-acetate.
Table 2: Recalculations of experimentally positioned compound composition of the monoterpenes in plants Artemisia balchanorum (in the tab. 1) to normalized mass fractions accordingly to the degree of monoterpene’s cyclization during the vegetation phases.
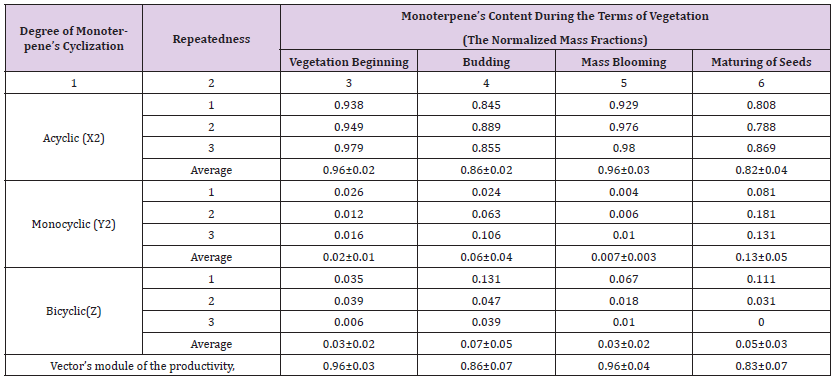
The maintenance of them increases in the vegetation beginning up to the phase budding or bloomings, and that is decreases up to the vegetation end. The maintenance of linalil ester (in the repeatedness) during the vegetation phases corresponds to 5.62%, 13.27%, 1.73%, 12.55%; that of neral – 4.68%, 4.44%, 7.24%, 6.26%; that of geraniol – 11.46%, 2.40%, 4.41%, 13.14%; that of geranial – 6.73%, 6.33%, 17.02%, 10.61%; that of geranyl-acetate – 12.80%, 5.18%, 11.38%, 2.81%. The quantitative maintenance of the acyclic monoterpenes in the extracts exceeds the maintenance of all other monoterpene’s groups. The greatest maintenance of that is observed during the vegetation phase beginning (51.33%) and during the phase budding (70.60%). The greatest maintenance of the mono- and bicyclic monoterpenes is observed during the fruiting phase (5.22% and 7.12% accordingly). Thus, for the first time one made the detailed studying of the qualitative and quantitative maintenance of monoterpen’s components in plants A.balchanorum during all vegetative phases. The further kinetic processing of the received experimental data was made according to the early modelling schema (Ustimenko [24]), which is used by us as is also for a plant cell level.
Numerous researches of monoterpene’s biosynthesis in the plants were carried out by the registration of a marked [2-14С]-mevalonate. [2-14С]-mevalonate is usually included in that part of the molecule which is formed immediately from IPP, and the insignificant part is included in that which is formed from DMAPP. So, the disposition of a radioactive label in bicyclic monoterpenes (Figure 1) shows that thujone
1. Contains 99% of the label in carbonyl group, and in camphor
2. The radioactive label settles down in 6th At the same time in the monocyclic monoterpene pulegone
3. The label is distributed between two positions which are in that part of the molecule, which was formed from IPP (Henson [27]).
To find-out the causes which have led to such disposition of the radioactive labels at the monoterpene’s biosynthesis we offered to include the isoprene, which always is present at plants (Khodakov [28]) in the schema of this biosynthesis. And it is known that the way of its formation is independent and it excludes the participation of IPP and DMAPP (Plemenkov [29]). Then it is possible to present the general schema of the monoterpene’s biosynthesis with the addition to it three intermediates (XОPP, YОPP and B), and the activated complexes and the cyclic products also (Figure 2). The formation of the corresponding cyclic recurrence of the molecular hydrocarbonic carcass descends during one stage by the combination of three initial products: isoprene (B), DMAPP (XОPP) and IPP (YОPP) at their linking together (Figure 2), where B, X and Y are the five-member hydrocarbon structures. As a result the label in bicyclic monoterpenes gets to 6-th position of camphor and to the carbonyl group of thujone. For monocyclic monoterpenes only one chemical structure is given (Figure 1), which is presented in the schema as two forms, which are strait on 180о about the axis (Figure 2), and after the oxidation in a meta position that leads finally to the same structure but marked twice. As an evident demonstration the molecule of pulegone is presented with a random distribution of the radioactive labels between two positions approximately on 50% (Figure 1).
For the acyclic monoterpenes there are no the reliable experimental data, which may indicate the presence in them of the radioactive label, and apparently their biosynthesis is carried out without the participation of IPP. In our scheme there is the single-stage of the initial products combination, in which the acyclic monoterpenes are formed by the combination of DMAPP with isoprene, the monocyclic monoterpenes – by the combination of IPP with isoprene, the bicyclic monoterpenes – by the combination of IPP with DMAPP. One explains the participience of isoprene in the formation of an acyclic monoterpenes by the existence of monoterpenes having an irregular structure, which were detected in a considerable quantity and in a structural diversity, because in the case of the single-stage combination it is necessary that the forerunner has four nucleophilic centres in this ctructure, and the molecule of isoprene possesses such quantity of the centres, and that leads to the formation of four groups of the acyclic monoterpenes (Figure 3). Therefore the choice of the isoprene’s molecule as the substrate is quite substantiated, and the carbocation of dimethyl-allyl is possible in this case as the electrofil agent. Menthanes (methyl-isopropyl-cyclo-hexanes) include hexatomic cycles as the more stable by the thermo-dynamics, which has in plants two basic structural analogues, namely: meta- and para-isomers pocessed the structures of 1-methyl-4-isopropyl-cyclo-hexane and 1-methyl-3-isopropyl-cyclo-hexane accordingly. The great mass of the menthane’s terpenes is the para-isomers (Figure 4).
The meta-isomer (sylvestrene) exists in the form of two optically active stereoisomers, admixture of that forms the racemate named by carvestrene. One supposes that it is formed as the artefact from 3-carene (Δ3-carene) in consequence of its destruction when the essential oil is produced by the method of steam distilling (Plemenkov [29]), but we suggests to consider it as the native plant substance, which is formed if the cascade cyclization of two intermediates Y and B take place. And, depending upon the orientation of B on terpene-synthetases its para-isomer as the basis of all known terpinenes in the nature (Figure 4) can be formed. It is defined that initial substances at the terpene’s biosynthesis are glucose and mevalonate (Rohmer [30-32]), which make the intermediates such as carbocations X and Y and further lead to all types formation of monoterpene’s hydrocarbons. Additional researches can conclude that the basic source of the biosynthesis is glucose, from which the IPP is formed by two ways, and that exists in a dynamic equilibrium with the DMAPP (Britton [33]). And only one label gets to a molecule of the bicyclic monoterpene from [14С] marked mevalonate (Figure 2), and that indicates on this distribution of the label only by one way of biosynthesis, hence, the second five-atomic molecule is formed by the parallel way of the biosynthesis. Thus, the assemblage of bicyclic monoterpenes results from the combination of molecules DMAPP and IPP by different biosynthesis ways (DXP and MVA accordingly).
It is possible to present the series of formed bicyclic monoterpene’s structures using the cascade cyclization form of five-atomic carbocations X and Y (Figure 5). The way of MVA-formation is realized in cytosols (earlier stages), endoplasmic reticulum (3-hydroxi-3-methyl-glutaril-KoA-reductase) and peroxisomes (posterior stages) (Carrie, et al. [34-41]). All ferments of the DXP-way are localized in the plastids (Hsieh [42,43]). The relative contribution of each way to determined structures of terpenes can differ essentially in various plants or in fabrics. To substantiate the dynamics of the monoterpene’s products accumulation in plants in a stream of substanses we composed the simplified schema, which explains the formation of a kinetic dependence using the data about the localization of cyclic products in a plant cell and the ways of their intermediate’s formations (Figure 6). It is known that two ways of biosynthesis are realized in the different parts of a cell divided by the plastid membrane. On each part of the cell its series of acyclic and monocyclic monoterpenes is formed. The biosynthesis of bicyclic monoterpenes is realized at the disposition of one intermediate (X) to assembly the molecule on the ferment through the plastid membrane. The penetrability of the membrane can affect on the kinetic constant of biosynthesis (k3) whatever of the ferment activity. We observed this effect at the definition of monoterpene’s biosynthesis singularities in the chemoraces of plants Artemisia scoparia, which have been described for the first time using the method of principal components (Zhigzhitzhapova, et al. [44]).
However, we applied own method of the research (Ustimenko [17]), which has allowed to find more deep particularities and to definite the essential differences between values of relative kinetic constants k3 for the chemo-races Artemisia scoparia produced in Bashkiria and that in Crimea (0.85 and 25.85). And the values of other constants of the biosynthesis remained at the same level in both chemo-races, and that indicates precisely on the influence of a specific factor on the biosynthesis of bicyclic monoterpenes in these plants. Such specific factor, in our judgment, is the penetrability of the plastid membrane for X. Schwender with employees (Schwender [45]) investigated the penetrability of plastid membranes, using a mevinoline as the high specific inhibitor of the mevalonate. As a result they reveal the characteristic way of IPP-biosynthesis in chloroplasts because the mevinoline does not penetrate through the membrane into chloroplasts and it does not block the biosynthesis of steroids in higher plants, therefore it does not influence to the formation of chlorophyll and the carotenoids in plastids. Similarly into chemo-races Artemisia scoparia one observes the limited penetrability of the plastid membrane for X, and that indicates on the separated ways of the intermediate’s biosynthesis during the formation of the bicyclic monoterpenes in a cell.
At the same time the kinetic constants of the acyclic and monocyclic monoterpene’s biosynthesis (kr1 and kr2) in both chemo-races coincide, and that confirms the existence of the complete series of the ferments at the biosynthesis of acyclic and monocyclic monoterpenes both in plastids, and in cytosol (Cunillera, et al. [6-9]). Monoterpenes are a part of some essential oils, which saturate the atmosphere around the producing plants. «In the system named plant-phytopathogene’s microorganisms monoterpene’s hydrocarbons can be considered as the constant acting factor by the primary nonspecific protection» (Kintya, et al. [16]). As a result, it is possible to assume that chemoraces are the hybrids of the investigated plants together the native flora, and which have taken place the natural selection in native conditions, where the biosynthetic mechanism was stable with the new kinetic constant, and that produced the qualitative and quantitative series of monoterpenes sufficiently to the ecological conditions. The combination of IPP with an isoprene molecule (Figure 2) leads to the formation of two symmetrically marked structures of monocyclic monoterpenes (pulegone), and it indicates on two-stage (at least) process, when the formation of an whole oxygen-containing molecule takes place, and the radio carbon disposition in two positions results from the non-selectivity of the subsequent oxidizing process for a complete hydrocarbon carcass, which is realized, apparently, after its carrier to peroxisome.
Thus, the increasement of a hydrocarbon carcass to form the higher terpenes is realized by the proper mechanism through the GPP-formation, and the formation of the monoterpene’s diversity descends from other way without its participation. Namely, process is realized by the ferment combination of phosphated substrates among themselves or any of them with the isoprene, which takes place by the mechanism of single-stage cascade cyclization. To define the kinetic and concentration interrelations between the bio-agents introduced in the schema (Figure 2), we compounded the simplified kinetic schema (Figure 6), and for that some admissions are made. So, the formation of the intermediates X and Y descends by two ways: from glucose (A) through a pyruvate or through a mevalonate (R) (Dewick [46]). The subsequent combination of X or Y with B leads to obtaining of monoterpene’s hydrocarbons having acyclic (X2) (Figure 3) and monocyclic (Y2) (Figure 4) structures formed by I-th series in plastids (through a pyruvate), formed by II-th series in cytosol and peroxisomes (through a mevalonate). The formation of bicyclic monoterpenes (Z) (Figure 5) is realized by the single-stage combination of Х and Y by different biosynthetic ways when Х penetrates through a semipermeable membrane and when it is combined by I-th way (DXP), and when Y is combined by II-th way (MVA), thereby linking both ways among themselves in the unique whole mechanism (Figure 6).
Such combinations will well be conformed to experimental data about the detection of one radioactive label in [2-14C]-mevalonic acid in the sixth position for camphor, thujone and that indicates to its appearance mainly of cause Y (Khodakov [28]). Besides it is known that the Y-formation descends by two ways (Pan, et al. [47]), and the appearance of this label descends only by the MVA-way. We supposed that the kinetic characteristics of acyclic and monocyclic monoterpene’s formation in both ways of the biosynthesis are the same. Initial conditions for the decision of the kinetic equations (1)– (3) are resulted in (Table 3). All processes descend on the ferments (terpene-synthases) corresponded to themselves kinetic constants (kri and k3). The introduced process is similar to known ferment assurance of diTPS (Gong, et al. [15]), which forms the diversity of the diterpene’s hydrocarbon carcass by the realisation of the cascade biosynthesis, but only from obtained acyclic (С-20) phosphated substrate. The graphic dependences between the characteristic parametres of the monoterpene’s biosynthesis (Figures 7 & 8) correspond to the solutions of differential equations with three variables (1). By the points on the graphs one indicated the experimental value, which in an optimum variant should coincide with the modelling curves or the received curves should come nearer to them more and more. The check for the correspondence of the modelling curves to experimental data according to Fisher's test of an adequacy estimated the 95% level of that for the modelling solutions (the algorithm of check was presented in the article (Ustimenko [17]).
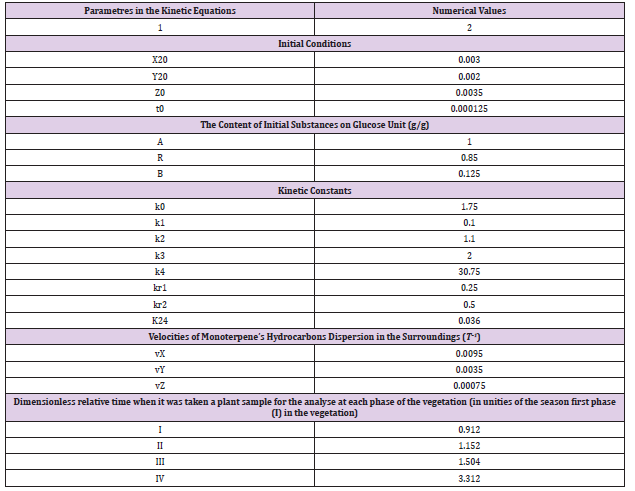
Table 3: Initial conditions for the kinetic equations (1)–(3) at their solution applying to the experimental data about the extracts from plants samples of the vegetable species Artemisia balchanorum Krasch.
As well the factor planes (Figure 8) open the relations between the kinetic stages of natural monoterpene’s biosynthesis in investigated plants by their velocities at each stage according to the kinetic scheme (Figure 6). As a result the individual biosynthesis particularities of each plant are obviously traced, and in the presented data there are obtained the direct acknowledgement of the general chemo-taxonomic indications in the investigated samples of plants according to work (Ustimenko [17]).
• The points indicate the received experimental data about the normalized concentrations (Table 3); the lines represent the solutions of the system of differential equations with three variables for the variable monoterpene’s normalized concentration:
1. Acyclic;
2. Monocyclic;
3. Bicyclic;
• The points designate the experimental values of the vector efficiency (Table 3); the lines represent the calculated values of the vector efficiency changes.
So, due to maintain the equilibrium concentrations of the carbocations Х and Y it is observed the prevalence of the non-mevalonate way of the biosynthesis from A over the mevalonate way from R (the value of the constant k0 is significant in excess of k1). The high-speed stage of the A-transformation to Х (reversible transferring is essentially retarded because of low value k24) is dominated. One observes the very high-speed stage for the expense of Х and Y on the Z-biosynthesis (high value of the constant k3). The expense of Х is completely compensated by its formation unlike the biosynthesis of Y2 and Z. That is the cause of non-uniform distribution of the remaining content of the detected monoterpene’s groups in the plants (the significant prevalence X2 over Y2 and Z). The stages with the participation of B are rate-defining in the biosynthesis of X2 and Y2 (low value of constants kr1 and kr2) that is undoubtedly caused by the low connatural bioactivity of corresponding terpene-synthetics. Thus, the quality and quantitative content of monoterpene’s hydrocarbons in the plants samples of species Artemisia balchanorum is detected during all phases of vegetation with the distribution of the components according to the degree of cyclization and with the definition of their group quantity properties.
The qualitative and quantitative maintenance of monoterpene’s hydrocarbons in plants of species Artemisia balchanorum is revealed during all phases of vegetation with the allocation of components according to the degree of ring formation and with the definition of their group quantitative characteristics. One offered the unique mechanism of the monoterpene’s hydrocarbons biosynthesis by the single-stage cascade combination of initial intermediates, which are added in the kinetic scheme combined two parallel ways of the process and which are realized in the various parts of the plant cell divided by the plastid membrane. One establishes the kinetic characteristics of the dynamics of monoterpene’s accumulation in plants according to the degrees of cyclization in the stream of substances using own theoretical and methodical approaches based on the proposed kinetic scheme. One found the common and specific particularities of the monoterpene’s biosynthesis in the plants samples of species Artemisia balchanorum and in chemoraces of species A.scoparia. One disclosed the essential influence of plastid membrane on the kinetic characteristics of the bicyclic monoterpene’s biosynthesis for chemoraces of plants in species A. scoparia and one confirmed the existance of whole series of the ferments at the formation of acyclic and monocyclic monoterpenes both in cellular plastids, and in cytosol of cells also.


