Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Nassime Zaoui*, Katia Babou, Amina Boukabous and Nabil Irid
Received: November 27, 2023; Published: December 18, 2023
*Corresponding author: Nassime Zaoui, Cardiology department, EHS Draa Ben Khedda Tizi-Ouzou Algeria
DOI: 10.26717/BJSTR.2023.54.008514
Introduction: Hypertrophic cardiomyopathy (HCM) is defined by the presence of “inadequate” myocardial hypertrophy because it develops in the absence of a cause of increased afterload (hypertension, aortic stenosis, subaortic membrane), an infiltrative pathology or a physical training. Alcohol septal ablation has become one of the therapeutic methods of choice in the event of failure of drug treatments, its aim is to reduce the gradient in the LV outflow chamber and consequently reduce the symptoms and improve the prognosis of patients.
Objective: To describe step by step the alcohol septal ablation (ASA), as well as the post-alcoholization evolutionary modalities of a series of 58 patients and to identify the factors of poor result (persistent gradient) at 3 months after ASA.
Method: this observational, retrospective and single-center study was conducted in a cardiology department, using a registry collecting clinical, biological and imaging data on 58 patients with HCM due to sarcomeric mutation suspected on echocardiography and confirmed on cardiac MRI candidates for alcohol septal ablation, with mean follow-up of 6 months. The patients were then divided into 2 groups according to the residual gradient in the LV outflow chamber at 3 months’ post-ablation (<50 mmHg VS >50 mmHg).
Results: 58 patients (32♂/26♀) aged 26 to 64 years with HCM were included, 100% of patients presented with LVH in ECG and 47 (81%) with bundle branch; The mean ejection fraction (EF) at baseline was 64.3% (58-72%) with a mean maximum LV thickness at 23 mm and mean maximum gradient at 88 mmHg. Treatment with beta-blocker (Propranolol 160-320) and/or Diltiazem (360-720 mg) was initiated in all patients before the ASA procedure. During the procedure we did not report any deaths, but we report one RV perforation by the temporary training probe with tamponade managed by cardiac surgery, VF managed by external electric shock and 3 complete AV block requiring the implantation of DDD-R Pacemakers Clinical, electrical and echocardiographic monitoring was performed every 3 months up to 6 months. A significant improvement in LV outflow residual gradient (<50 mmHg) was observed in 45 patients (77.59%). Gender, age, ECG, LVEF and quantity of alcohol injected < 2ml do not seem to influence the evolution of the gradient, LV maximum thickness >30mm, initial gradient >135 mmHg seem to be good predictors of poor result avec ASA therapy (P <0.05).
Discussion: We report a zero-mortality rate and a morbidity rate of 8.62% which is consistent with the literature data. The factors of poor result after ASA therapy are LV maximum thickness >30mm and initial gradient >135 mmHg (P <0.05).
Conclusion: The alcohol septal ablation technique is fast, effective and safe, the benefits are comparable to those observed with surgical myectomy in terms of functional class, exercise capacity and gradient regression with less morbidity.
Keywords: Patient Series; Hypertrophic Cardiomyopathy; Alcohol Septal Ablation; Lvot Gradient; Ethanol
Abbreviations: ASA: Alcohol Septal Ablation; ECG: Electrocardiogram; HCM: Hypertrophic Cardiomyopathy; LA: Left Atrium; LV: Left Ventricle; LVOT: Left Ventricle Outflow Tract; MRI: Magnetic Resonance Imaging; RV: Right Ventricle; TTE: Transthoracic Echocardiography
Hypertrophic cardiomyopathy (HCM) is the most common monogenic heart disease, affecting approximately 1 in 500 individuals in the population [1]. Etiology is familiar in the majority of cases with an autosomal dominant type of transmission with variable penetrance [1,2]. HCM is defined by the presence of an "inadequate" myocardial hypertrophy because it develops in the absence of a cause of increased afterload (hypertension, aortic stenosis, sub-aortic membrane), an infiltrative pathology or physical training [2]. Most often asymmetrical and preferentially affecting the septum, this hypertrophy is accompanied in almost two-thirds of cases by dynamic sub-aortic obstruction of the left ventricular outflow chamber with the mitral valve (systolic anterior motion or SAM). This obstruction is both the consequence of the narrowing of the outflow chamber by septal hypertrophy but also of a miss-positioning of the mitral valve. This is called Hypertrophic and Obstructive Cardiomyopathy (CMHO) [1,3]. Obstruction: present at rest in 50% of cases and only after provocation maneuvers in the other half of cases (Valsalva maneuver, effort) is the cause of a pressure gradient between the left ventricle and the aorta, and therefore a pressure overload for the left ventricle [3]. This pressure overload is at the origin of the symptoms classically encountered, namely dyspnea and exertional angina, pre-syncope or even syncope on exertion [3]. A subaortic gradient of more than 50 mmHg (measured at rest or after provocation) is considered a gradient with prognostic value and justifying treatment if associated with symptoms [4].
The medical treatment of obstructive forms is based on the administration of negative inotropic substances and/or substances likely to promote myocardial relaxation such as beta-blockers, calcium antagonists and disopyramide taken alone or in combination [5,6]. For the many patients who become refractory or intolerant to these treatments, two interventions can be proposed to them to remove the obstruction: surgical myotomy-myectomy of the septum or percutaneous alcoholization of the septum [7]. If surgical myectomy remains the reference method, alcohol septal ablation of the myocardium by percutaneous approach has become one of the treatments of choice in the therapy of refractory Hypertrophic Obstructive Cardiomyopathy. It consists of identifying by coronary angiography the septal artery feeding the hypertrophied basal septum, injecting a dose of 95% alcohol between 1 and 5 cc [7,8]. This creates a chemical infarction, a technique that was used in the past for the treatment of certain tumors. The effects are not immediate and usually take 2-3 weeks to appear [8]. There is then a gradual decrease in the thickness of the necrotic myocardium, the gradual disappearance of the obstruction and the improvement/disappearance of the symptoms [6-8].
Context and Justification
Several meta-analyses have paralleled myomectomy and septal alcoholization (approximately 20,000 patients) and have demonstrated that surgery is clearly challenged by septal alcoholization which is preferred by the patient with a similar improvement in functional status: at least 80% of patients improve their gradient and their symptoms at the cost of an identical mortality of around 2%. Making alcohol septal ablation a technique to know, master, improve and compared to literary registers [7,8].
Objective
Describe step by step the alcohol septal ablation (ASA), as well as the post-alcoholization evolutionary modalities of a series of 58 patients and identify the factors of poor result (persistent gradient) at 3 months after ASA.
Design and Context of the Study
this observational, retrospective, and single-center study was conducted in a cardiology department, using a registry collecting clinical, biological and imaging data on patients with HCM candidates for alcohol septal ablation from January 2017 to January 2023.
Participants
This study included all patients with hypertrophic cardiomyopathy due to sarcomeric mutation suspected on echocardiography and confirmed on cardiac MRI admitted in our department for ASA (total of 58 patients). Patients with amyloidosis, Fabry disease, etc. were excluded from the study (n= 12). Patients were controlled every 3 months (clinically, ECG and TTE) and divided into 2 groups according to the residual gradient in the LV outflow chamber at 3 months’ post-ablation (<50 mmHg VS >50 mmHg) and compared to identify factors predicting poor result after ASA. We did not deplore any loss of sight or death during the follow-up period. All participants have given their consent to participate and share the results of the work.
Variables and Assessment
Symptoms and clinical examination were assessed at each consultation.
ECGs were performed on 12-lead machines, assessing:
• Heart rate
• Heart rhythm
• PR, QT and QTcB intervals (calculated according to the Bazett formula)
• QRS axis and duration
• Presence or absence of atrio-ventricular blocks or bundle branch blocks
The following echocardiographic parameters were measured on a Vivid S6 ultrasound system:
• End diastolic and end systolic diameters of the LV • LV ejection fraction calculated by Teicholtz • Septal thickness • Sub-valvular ejection gradient at rest and after provocation maneuver• Presence or not of mitral regurgitation and its severity
The MRIs were performed in reference cardiac MRI centers on GE 1.5 Tesla machines. Coronary angiograms and ASA procedure were performed on Cath Lab GE Optima. The occurrence of local (hematoma, arteriovenous fistula), cardiac or systemic complications in perioperative or early postoperative period, and of course the appearance of complete atrioventricular blocks were noted in the medical records.
Study Bias Management
Selection bias: To reduce these biases and to make the study population as representative as possible of daily practice, we did not limit the origin of the patients whose recruitment was successive, most often on the advice of their referring doctor.
Verification Bias
All patients included in the study received the mandatory reference test (MRI to confirm sarcomeric HCM).
Interpretation Bias
A double-blind determination by two echocardiographers and an average of the results if the difference is <10% in maximum gradient was performed at baseline and every 3 months in all patients.
Statistical Analysis
All the data were collected using the EPI-INFO 7 software. The results were expressed as a percentage for the qualitative variables and as a mean ± standard deviation (SD) for the quantitative variables. Bivariate analysis of all the parameters according to the evolution of the maximum LVOT gradient were carried out according to the Fisher test for the qualitative variables and the Student test for the quantitative variables.
P value <0.05 was considered statistically significant.
Alcohol Septal Ablation Technique
Here we describe all the techniques and equipment needed to perform an alcohol septal ablation procedure.
This procedure begins with a good selection of patients (clinical, EKG, TTE and cardiac MRI to confirm the sarcomeric form and optimal medical treatment) (Figure 1). Then, like any coronary angiography procedure, an arterial approach will be taken (or double approach in order to assess the invasive LV-Aorta gradient pre and post-procedure), a complete coronary angiography is performed if necessary then a guiding catheter with excellent support is chosen according to the anatomy of the left network (EBU or AL) and a guide 0.014 is placed in a septal artery (Figure 2). A vein puncture with placement of a temporary pacemaker may be necessary if pre-existing complete or incomplete let bundle branch block. An OTW balloon adapted to the diameter of the septal artery is introduced then inflated at low pressure (6 to 8 atm) in this septal artery, an injection of iodine product is made through the guiding catheter to confirm that the balloon excludes the septal then through the balloon to confirm the absence of fistula of this septal artery.
The last step is to inject ultrasound contrast into this septal and to confirm in TTE that the selected artery corresponds to the correct part of the septal muscle to be ablated. Once the correct septal artery has been identified, a quantity of Ethanol 95% is injected over 5 minutes followed by 5 minutes of washing with serum then a waiting time of 5 minutes before deflating the balloon and evaluating the invasive and echocardiographic gradient and to remove the material (Figure 3). The quantity of Ethanol to be injected is calculated according to two methods: 0.08 cc of Alcohol for every mm of septum or 01 cc of alcohol for every mm of septal artery.
Participants and Descriptive Data
Our study included 58 patients (32 men and 26 women) aged from 26 to 64 years with sarcomeric HCM suspected on echocardiography and confirmed by cardiac MRI. All patients had dyspnea (class 3 or 4 NYHA) under maximum tolerated medical treatment with beta-blocker (Propranolol 160-320) and/or Diltiazem (360-720 mg) for a minimum of 6 months. All patients presented with LVH in ECG and 47 (81%) with bundle branch; The mean ejection fraction (EF) at baseline was 64.3% (58-72%) with a mean maximum LV thickness at 23 mm (18 to 33) and mean maximum gradient at 88 mmHg after Valsalva maneuver (50-139 mmHg). We calculated the quantity of alcohol needed according to the two techniques and started for each patient to inject the minimum amount and completed towards the maximum amount in case of incomplete result during the immediate perprocedural evaluation. The mean quantity of Ethanol injected in our study was at 2.4 +/- 0.6 cc (1.8 to 3.0 cc). During the procedure we did not report any deaths, but we report one RV perforation by the temporary training probe with tamponade managed by cardiac surgery, VF managed by external electric shock and 3 complete AV blocks requiring the implantation of DDD-R Pacemakers.
Analysis
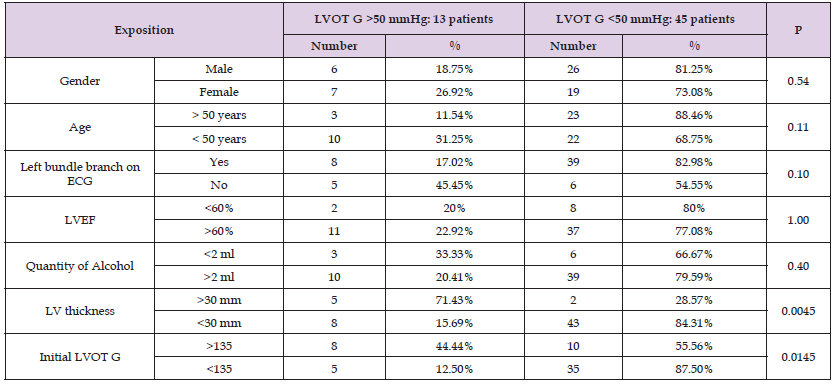
Clinical, electrical, and echocardiographic monitoring was carried out during the procedure, at 48 hours and then every 3 months for all the patients up to 6 months. A significant improvement in LV outflow residual gradient (<50 mmHg) was observed in 45 patients (77.59%). We divided then our patients into 2 groups according to the residual gradient in the LV outflow chamber at 3 months’ post-ablation (<50 mmHg VS >50 mmHg) and compared these two groups to identify factors predicting poor result after ASA (Table 1):
• Gender does not seem to influence the evolution of LVOT gradient at 3 months (♂: 6/32 VS ♀: 7/26, P at 0.54).
• Age > 50 years does not seem to influence the evolution of LVOT gradient at 3 months (> 50 years: 3/26 VS <50 years: 10/32, P at 0.11).
• Left Bundle Branch on ECG does not seem to influence the evolution of LVOT gradient at 3 months (LBB+ 8/47 VS LBB- 5/11, P at 0.10).
• LVEF do not seem to influence the evolution of the gradient (LVEF<60%: 2/10 VS LVEF>60%: 11/48, P at 1.00).
• Quantity of alcohol injected < 2ml do not seem to influence the evolution of the gradient (Qt <2 cc: 3/9 VS Qt>2cc: 10/49, P at 0.40).
• LV maximum thickness >30mm is associated with poor result on LVOT gradient at 3 months (> 30mm: 5/7 VS <30mm: 8/51, P at 0.0045) (Figure 4).
• Initial gradient >135 mmHg seems to be good predictors of poor result avec ASA therapy (G>135 mmHg: 8/18 vs G<135 mmHg: 5/40, P at 0.0145) (Figure 5).
Table 1: Influence of different factors on the evolution of LVOT gradient 3 months after alcohol septal ablation.
Limitation
The main limitation of this study is the small size of the collective (n =58), which does limit to have statistically significant results. Through this we have rare complication rates, compared to the data in the literature obtained in larger collectives. The retrospective profile of the study also has a limitation. Indeed, some examinations are missing or incomplete, thus limiting the amount of data available.
Main results and Interpretation
We report a zero-mortality rate and a morbidity rate of 8.62% which is consistent with the literature data.
The factors of poor result 03 months after ASA therapy are LV maximum thickness >30mm and initial gradient >135 mmHg (P <0.05).
Alcohol septal ablation has become an attractive alternative to surgical myomectomy in symptomatic patients with obstructive hypertrophic cardiomyopathy. The selection of candidates must be rigorous and the procedure must be entrusted to an experienced center, combining interventional cardiologists and echocardiographers. Septal alcoholization is preferred in case of subaortic obstruction, favorable coronary anatomy, and absence of associated anomaly of the subvalvular mitral apparatus. The alcohol septal ablation technique is fast, effective and safe. Per-procedural contrast echocardiography can easily identify the septal branch to be alcoholized. The benefits of ASA are comparable to those observed with surgical myectomy in terms of functional class, exercise capacity and gradient regression for septum thickness < 30 mm. Morbi-mortality, observed in the short and medium terms, is globally equivalent to that of surgery. The major complication is dominated by the occurrence of complete atrioventricular block requiring the implantation of a permanent pacemaker, a complication that has been in sharp decline since the ultrasound-guided technique became widespread.
What we Know
The alcohol septal ablation technique is fast, effective, and safe. Its morbi-mortality is globally equivalent to that of surgery. The own limitation is the existence of anatomical contraindication as fistula or no adequate septal artery.
What this Study Adds
The results of alcohol septal ablation technique are less good on septal thicknesses > 30mm and starting gradients >135 mmHg. These situations could be in the future preferential indications for immediate surgical treatment.
Ethics Committee
The ethics committee (represented by the local scientific council of our hospital) has given its agreement to carry out this work and share the results here.
Informed Consent
All patients gave their consent for participation and publication of the results of this study.
The hospital's ethics committee has given its consent to carry out this study and share the results under number 003/23. All participants gave their informed consent to participate retrospectively in this study and to share the results.
All participants in this work consented to the sharing and publication of data and results.
The datasets used and analyzed during this work are available from the corresponding author on reasonable request.
The authors declare that they have no competing interests.
Not applicable.
NZ was responsible for the design of the study, participated in the realization of echocardiographies and alcohol septal ablation procedures, interpreted the results and participated in the writing of the manuscript. NI participated in the realization of echocardiographies and alcohol septal ablation procedures and in the writing of the manuscript. KB participated in the realization of echocardiographies and carried out the analysis and statistical tests. AB participated in the analysis and interpretation of the results and the realization of echocardiographies. NI participated in the analysis and interpretation of the results and the realization of echocardiographies.
We thank our paramedics who participated in the explorations carried out in this study and our medical secretaries who ensured the archiving of the patients’ data.
No.


