Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Mohamed F Balaha1,2*, Rana M Aldossari3, Alhussain H Aodah4 and Aftab Alam5
Received: December 01, 2023; Published: December 12, 2023
*Corresponding author: Mohamed F Balaha, Department of Clinical Pharmacy, College of Pharmacy, Prince Sattam Bin Abdulaziz University, Al-Kharj 11942, Saudi Arabia
DOI: 10.26717/BJSTR.2023.54.008497
Background: A range of agents are prescribed for the treatment of hepatic dysfunction or as a tonic, which serve to safeguard the liver, stimulate appetite and growth, and regulate gastrointestinal functions. However, there is inadequate empirical support regarding their safety and efficacy. More research needs to be conducted to ascertain how effective these agents are in remedying liver ailments.
Material and Methods: the present Study explored whether Silymarin (SIL) with resveratrol (RES) at different dose of (SIL 50 +RES 50; SIL 50 +RES 100; SIL 100 +RES 100) can shield against acute paracetamol (PCM)-related hepatotoxicity in rats exemplifying possible advantages of such compounds towards attaining sustained organ functionality. In the study, an oral dose of PCM (1g/kg) on day 8 was administered to induce hepatotoxicity. Liver enzyme levels were monitored, encompassing ALT, AST, total cholesterol, total protein and triglycerides. Furthermore, glutathione, superoxide dismutase (SOD), NO, catalase, TBARS, Il-6, and TNF-α in the liver as well the histopathological changes were assessed.
Results: the data of the present study reported that SIL +RES can effectively treat hepatotoxicity in rats caused by PCM in a dose-dependent mannar, as revealed by restoration on hepatic anti-oxoident activity, suppression of hepatic inflammation and abbrogation of histopathollgical changes induced by PCM.
Conclusion: The results of the study exhibited that SIL +RES can effectively treat hepatotoxicity in rats caused by PCM at three different dosage levels. Nevertheless, it implies that optimization of dosing may be required for clinical purposes to achieve maximum benefits.
Keywords: Silymarin; Resveratrol; Paracetamol; Hepatoprotective; Il-6; TNF-α; Oxidative Stress
Abbreviations: SIL: Study Explored Whether Silymarin, RES: Resveratrol, PCM: Paracetamol, SOD: Superoxide Dismutase, NAPQI: N-Acetyl-p-Benzoquinone imine, GSH: Glutathione, NAC: N-Acetylcysteine, SIL: Silymarin, RES: Resveratrol, ALT: Alanine Aminotransferase
Paracetamol (PCM), or acetaminophen, effectively manages medical conditions by relieving pain and reducing fever. It's a healthcare provider's preferred choice for patient relief from discomfort caused by different illnesses. Improper use of PCM can have serious consequences, even though it's usually considered safe when taken as directed [1]. Overdose can be fatal due to liver damage. In some cases, the affected individual may need an urgent liver transplant to prevent loss of life. Therefore, individuals need to exercise caution when taking PCM and follow the prescribed dose closely [2]. Monitoring one's health for any possible symptoms and seeking the assistance of medical professionals who demonstrate genuine concern towards their patients is also important to maintain caution [3]. In the United States, PCM remains a major cause of overdose-related liver failure and death accounting for 50 percent of all reported cases and approximately 20 percent of liver transplant cases [4]. The liver's vulnerability to harm from one of PCM's metabolites, N-acetyl-p-benzoquinone imine (NAPQI), becomes particularly pronounced at high dosages. This risk can be exacerbated by alcohol consumption and potential hunger-triggered cytochrome P-450 activation, which intensifies NAPQI production and raises the likelihood of liver damage [5]. PCM-induced hepatotoxicity emerges when concentrations of the metabolite NAPQI reach hazardous levels. Such situations often arise from overdose or excessive use, overwhelming the clearance mechanisms and leading to NAPQI accumulation and subsequent toxicity [6,7]. The body's swift detoxification of this potent metabolite involves conjugating it with hepatic glutathione and eliminating it through urine [8]. Nevertheless, when a significant quantity of PCM is ingested, the glutathione (GSH) reserves become depleted due to NAPQI accumulation resulting from saturation in the glucuronidation and sulfation metabolic pathways. This depletion of GSH leads to oxidative stress as there isn't enough glutathione to counteract NAPQI's toxic effects [9].
The consequences of PCM toxicity encompass oxidative stress, stemming from an imbalance between reactive oxygen species and antioxidants within cells. This oxidative stress results in liver damage characterized by ischemia, necrosis, and apoptosis, which triggers gene expression changes and culminates in severe liver impairment [10]. Various treatments are available to address PCM-induced hepatotoxicity, encompassing N-acetylcysteine (NAC), cytochrome P450 inhibitors, and glutathione supplementation [11,12]. NAC, recognized as the foremost and most effective therapy, operates by reinstating glutathione levels and augmenting NAPQI scavenging, thereby averting PCM overdose-induced liver damage [4,12-14]. Additionally, cytochrome P450 inhibitors can thwart PCM activation into its toxic form. Another approach involves using glutathione supplementation to counter PCM-induced hepatotoxicity, as it replenishes depleted glutathione levels [15,16]. Existing treatments for PCM-induced hepatotoxicity have some limitations like NAC has limited efficacy in preventing liver damage caused by PCM overdose [12], regular monitoring of risk factors after getting treatment [17], and limited prevention options after PCM overdose is still not well-established [18]. The limitations of existing therapies for PCM-induced hepatotoxicity have led to the exploration of herbal therapies. Although some herbal medicines have been shown to exacerbate PCM-induced hepatotoxicity, others have been found to have hepatoprotective effects against PCM-induced hepatotoxicity [19].
For example, psoralen, a common phytochemical in herbal medicines, was shown to synergistically enhance the toxicity of PCM [8]. On the other hand, some herbal medicines have been found to have hepatoprotective effects against PCM-induced hepatotoxicity. For instance, the methanol extract of Agave americana leaves has been found to have a hepatoprotective effect against PCM-induced hepatotoxicity in rats [20]. Apart from a single component, a multi-herbal combination consisting of Andrographis paniculata, Phyllanthus amarus, and Curcuma longa has been found to have a hepatoprotective effect against PCM-induced hepatotoxicity in rats [8]. Silymarin (SIL) is a flavonoid mixture extracted from the Silybum marianum (milk thistle) plant. It contains various flavonolignans, with SIL being the major one [21,22]. Silybins are the major constituents in SIL with almost 70–80% abundance and are accountable for most of the observed therapeutic activity [23]. SIL has been found to exert antioxidant [24], hepatoprotective [25], cardioprotective, and anti-inflammatory [21]. Whereas resveratrol (RES) is a naturally occurring phytochemical present in wine, grapes, berries, chocolate, and peanuts with the major presence of RES oligomers as phytoconstituents [26,27]. It exhibits numerous pharmacological properties such as antioxidant, anti-inflammatory, antidiabetic, and neuroprotective activities [28]. While the individual therapeutic effects of SIL and RES have been extensively studied, there is a research gap concerning their combined potential as an alternative therapy. Previous research has focused on their individual attributes, such as SIL's antioxidant and hepato-protective properties, and RES's various pharmacological activities. However, their combined effects and their possible synergistic benefits remain relatively unexplored.
Building on the concept of combination therapies, the current study hypothesizes that the synergistic application of SIL and RES could offer enhanced therapeutic effects compared to their individual administration. In pursuit of this hypothesis, our study seeks to elucidate the potential molecular mechanisms underlying the synergy between SIL and RES. By investigating their combined effects on antioxidant pathways, inflammatory responses, and overall liver function, we aim to provide a comprehensive understanding of how this combination therapy could offer improved therapeutic efficacy. Ultimately, these insights could pave the way for innovative strategies in addressing hepatotoxicity and may have broader implications for the development of synergistic therapies in other medical contexts.
The Drugs and chemicals
The Resveratrol was purchased from Sigma Aldrich Co., Saint Louis, Missouri, USA. PCM was procured from Sigma, St. Louis, MO, USA. However, SIL was purchased from Sigma Co. New Delhi, India. All other chemicals used in this study were of analytical grade. Polyethylene glycol 400 (PEG 400) was procured from Sigma-Aldrich, USA.
Animals
To experiment, male Wistar rats weighing between 250-275 g were bought from a local breeder. The rats received unlimited access to regular laboratory food and tap water throughout both the acclimatization and experimental phases. To maintain ideal conditions, with a stable temperature of approximately 30°C with humidity of 60 ± 10 % with a regular 12 h light/12 h dark cycle. The scientific methods used in this research adhere to ethical standards and protocols for the welfare of laboratory animals established by the Standing Committee on Bioethics Research " Prince Sattam Bin Abdulaziz University's (PSAU), Al-Kharj, kingdom of Saudi Arabia, approval no. SCBR/024/2022.
Experimental Design
The study involved the random selection of animals into different groups and each group consisted of 6 animals. Accurate dosage volumes were subsequently administered based on an individual's weight; details about this are comprehensively outlined in Table 1 [10,29]. We utilized a USFDA-approved solvent to dissolve both silymarin and resveratrol. Specifically, we solubilized PEG-400 at a concentration of 10 mg/mL.
Collection of Blood for Biochemical Estimations
At the end of the study (at 10 AM), the rats were anesthetized using thiopental sodium (120 mg/kg, administered via intraperitoneal injection). Blood samples were obtained via retroorbital puncture and then allowed to naturally coagulate for thirty minutes at a controlled room temperature. Following this, the samples were subjected to centrifugation at a speed of 2000g for a duration of ten minutes. The uppermost layer, characterized by its distinct yellow pigment, was carefully extracted through pipetting, taking care to avoid disturbing the underlying white buffy layer. The obtained serum was then stored at a temperature of -20 ℃ for subsequent assessment of various biochemical parameters [30].
Preparation of Tissue Homogenates
The rats’ livers were removed and placed in a saline solution with heparin (0.16 mg/ml) to avoid clotting. Each liver was separated into two equal sections, where the first part underwent longitudinal slicing (2-4 mm thickness). These slices were preserved in 10% formalin buffered with phosphate for further examination of their tissue structure. The second portion was promptly submerged under liquid nitrogen at -80 ℃ to determine other biochemical factors [30].
Percentage Hepatotoxic Protection
The significance of the hepatoprotection formula lies in its ability to assess drug effectiveness in mitigating or preventing liver damage, which is crucial for drug development. Essentially, the formula compares the occurrences of liver toxicity between patients who underwent treatment and those who did not undergo any form of medication. Percentage hepatotoxic protection was determined using the formula below [31]:
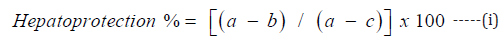
where a, b, and c are the mean ± SEM of PCM, SIL+RES, and control, respectively.
Estimation of Markers of Liver Injury
Aspartate Aminotransferase (AST): The technique for determining AST activities followed the instructions specified in the kit manual purchased from Biorbyt Ltd., Cambridge, UK. The assay involved adding 50 µL of diluted glutamate standards, positive control, or samples to each well of a 96-well plate; and then mixing with 150 µL of reaction reagent which resulted in a thorough mixture content per well. After incubating the contents at an optimal temperature (37℃) for a half-hour duration, an optical absorbance reading was taken at 450 nm wavelength through a spectrophotometric plate reader (Stat Fax 2100 automated plate reader, Fisher Bioblock Scientific, BP., Illkirch Cedex, France). Finally, the AST activity relationship function provided in kit manual could be used to calculate AST value performance. AST activity is expressed in IU/L [32].
Serum Alanine Aminotransferase (Alt): To determine serum alanine aminotransferase (ALT) activities, the protocol described in the kit manual, purchased from Biorbyt Ltd., Cambridge, UK, was followed. Firstly, 50 µL of the diluted pyruvate standards, positive control, or samples was added to each well of a 96-well microtiter plate. Then, 100 µL of the prepared Reaction Reagent was added to each well and mixed the well contents thoroughly. Immediately after mixing, the absorbance of each microwell was taken using on a spectrophotometric microplate reader using 540-570 nm absorbance (Stat Fax 2100 automated plate reader). ALT activity is expressed in IU/L [32].
Total Protein: To measure the amount of protein in a sample, various test tubes labeled Blank, Standard, and Tests were utilized. Next, 2.5 mL of Protein Assay Solution was introduced to each tube before adding either Sodium Chloride or Protein Standard Solution depending on the label assigned. A Test-tube received 50 µL of the actual specimen being tested. The contents for each flask were thoroughly mixed and allowed to rest for about two minutes until stable color appeared; absorbance values (A) were then measured using Biosystems semi-automated analyzer, BTS-350, Barcelona, Spain, against that observed from blank readings at 595 nm wavelength. The protein concentration of the samples was calculated by using following formula [33]:
Protein concentration (g / dL) = ( A Test ) × Concentration of Standard / (Standard ) --(ii)
Creatinine Level: Prepare the Working reagents and standard reagents as mentioned in the protocol of a colorimetric assay kit purchased from Crystal Chem., Busse Rd., Elk Grove Village, IL., USA. Read the absorbance (A1) after 30 seconds and after 90 seconds (A2) of the sample addition at absorbance 492nm using Biosystems semi-automated analyzer, BTS-350. The creatinine level of the samples was calculated by using following formula and expressed in mg/dL.
ΔA = A2 − A1
Creatinine (mg / dL)in sample = (ΔA sample − ΔAblank ) / (ΔA standard − ΔA blank ) (iii)
Urea Level: Prepare the Working reagents and standard reagents as mentioned in the protocol of a colorimetric assay kit purchased from Arbor Assays, Eisenhower Place, Ann Arbor, Michigan, USA. Read the absorbance (A1) after 30 seconds and after 4 minutes (A2) of the sample addition at absorbance 578nm using Biosystems semi-automated analyzer, BTS-350. Multiply the results with dilution factor 20. The creatinine level of the samples was calculated by using following formula and expressed in mg/dL.
Urea level (mg / dL)in sample = ( Absorbance sample) / ( Absorbance standard ) X standard conc --(iv)
Glucose and Lipid Levels: Prepare the Working reagents and standard reagents as per protocol and calculate the glucose level using GOD-POD method at wavelength 510 nm using Erba diagnostic kits. Similarily, for the estimation of lipid, we used the Erba diagnostic kits. Prepare the Working reagents and standard reagents as mentioned in the protocol and measure the absorbance at 505 and 525 using Biosystems semi-automated analyzer, BTS-350. Measure absorbance of the sample Asample and Astandard. The glucose and lipid level of the samples was calculated by using following formula and expressed in mg/dL.
Lipid level (mg / dL)in sample = ( Absorbance sample) / ( Absorbance standard ) X standard conc -(v)
Nitric Oxide (No) Content: To measure the synthesis of nitric oxide, we determined the concentration of total nitrate/nitrite products using colorimetric assay kits bought from MyBioSource Inc., San Deigo, CA, USA following the manufacture instructions and analyzed the absorbance level at 540 nm wavelength using Biosystems semi-automated analyzer, BTS-350.
Estimation of Liver Oxidative Stress Parameters
TBARS: It is possible to analyse the concentration of TBARS present in liver homogenate by measuring malondialdehyde. For this purpose, a colorimetric assay kits bought from MyBioSource Inc., San Deigo, CA, USA following the manufacture instructions and analysed at 530-540 nm using Biosystems semi-automated analyzer, BTS-350. MDA, which forms as a result of lipid oxidation, serves as the primary target for detection while secondary compounds are also included within the scope of TBARS assay. With detection sensitivity reaching up to 0.1 µM MDA in test samples, TBA must be synthesized using acetic acid and introduced into said samples during experimentation process. To assess lipid peroxidation and oxidative stress in biological samples, the TBARS assay is an indispensable tool used across various fields, including biochemistry, nutrition, and toxicology. In this assay procedure, the liver homogenate samples were incubated under controlled temperature conditions for a specific time duration before measuring their absorbance at 530-540 nm. The concentration of TBARS was then accurately determined by establishing a standard curve using known MDA concentrations to compare with test sample absorbances.
Reduced Glutathione Content: Glutathione is essential in maintaining cell balance and protecting against stress by capturing free radicals [34]. It's abundant in the liver and comes as either thiol- reduced glutathione (GSH) or disulfide-oxidized GSSG depending on oxidation [35]. Its levels are vital for gauging cellular redox state, assessing damage from stress, monitoring drug effects, and age-related diseases [14,36]. Measuring glutathione offers insights into liver antioxidative capacity that influences processes positively. In order to conduct the GSH examination, we adhered to the manufacturer's prescribed protocol of a colorimetric assay kits bought from MyBioSource Inc., San Deigo, CA, USA, which entailed introducing a designated quantity of the GSH substrate into our test specimens; subsequently allowing for incubation and further addition of an indicator agent. The enzymatic cycling method is utilized by the GSH Assay Kit in the presence of both GSH and a chromophore. By catalyzing the reduction of the chromophore, a long-lasting product is generated and can be monitored over time at A450 nm using Biosystems semi-automated analyzer, BTS-350. As a result, the quantity of GSH present in the sample can be accurately determined by analyzing its absorbance. The sensitivity and specificity of the assay kit are significantly high, as it is not influenced by GSSG interference.
Catalase in Liver Tissue: The Catalase Kit from MyBioSource Inc., San Deigo, CA, USA was for detecting catalase activity in biological samples. The kit includes a 96-well plate, catalase standard (100 Unit/mL), substrate, and more components to perform the assay. To ensure accuracy, it is recommended to use a bovine catalase standard in order to establish a benchmark for the assay. By generating a reliable standard curve, all subsequent samples were measured against this baseline with greater precision and confidence. Samples are diluted in the provided assay buffer and added to the wells of a half area clear plate. After the addition of hydrogen peroxide to individual wells, the plate is left to incubate at room temperature for a duration of 30 minutes. To detect color, a colorimetric detection reagent was incorporated into the solution and then diluted with horseradish peroxidase. Following this, it was left to incubate at ambient temperature for a duration of 15 minutes. In the presence of hydrogen peroxide, the substrate is converted into a pink-colored product by reacting with HRP. The wavelength of 560 nm was used to obtain a reading of the colored product. The presence of higher levels of catalase in the samples leads to a reduction in hydrogen peroxide concentration and consequently, a decrease in the formation of pink product.
Sod Assay: The SOD Activity Assay Kit from MyBioSource Inc., San Deigo, CA, USA is a fast and easy test to measure Superoxide Dismutase activity. The SOD Activity Assay Kit is an innovative colorimetric assay that offers a compelling solution to quantitatively evaluate the degree of SOD activity in numerous types of samples including cell culture media, cell lysate, other biological fluids, plasma, serum as well as tissue extracts and urine. The kit comprises a 96-well plate, superoxide dismutase standard (1 Unit/vial), substrate, and other necessary components for conducting the assay. The test detects various SOD activities and needs a 96-well microplate reader that can read optical density at 450 nm. The SOD Activity Assay Kit works by measuring the reduction of tetrazolium salt using superoxide radicals created from xanthine oxidase and hypoxanthine. In each well, 10 μL of either standards or diluted samples was supplemented. Then, 1X substrate amounting to 50 µL and 1X Xanthine Oxidase measuring 25 µL were introduced into the wells. After that, the mixture was heated at room temperature for around twenty minutes. It is worth noting that bright yellow specimens can affect the high sensitivity format assay and necessitate a blanking step before adding in the enzyme solution (i.e., Xanthine Oxidase). For this purpose, plate had its absorbance level measured beforehand by using chromogenic detection reagent at a wavelength of initially set value equal to or greater than of 450 nm. The strength of the hue is directly related to the superoxide dismutase activity found in the specimen.
Interleukin-6 (IL-6): A volume of 100μL of the sample was introduced into the wells and left to incubate for a period of 90 minutes at a temperature of 37°C. The liquid was then promptly disposed off, following which a working solution containing Biotinylated Detection Antibody in an amount measuring 100μL was added to each corresponding well. After a 60-minute incubation at 37°C, the plate underwent three washes. The working solution consisting of 100μL HRP conjugate was added and allowed to undergo an additional 30-minute incubation at 37°C. The plate underwent five rounds of washing before the addition of 90μL substrate reagent. The mixture was incubated at a temperature of 37°C for a duration of 15 minutes, after which 50μL stop solution was incorporated. Reading was taken at 450nm immediately. Results was calculated according to Abnova Co., Taipei, Taiwan, IL-6 Elisa Kit.
Hepatic TNF-α: Hepatic TNF-α ELISA Kit purchased from MyBioSource Inc., San Deigo, CA, USA is intended for measuring TNF-α levels in various biological samples such as serum, plasma, and other fluids through the application of sandwich ELISA methodology. The kit comprises a microplate coated with an antibody that specifically binds to the protein under examination. The procedure for the Hepatic TNF-α ELISA Kit from Elabscience involves adding 100μL of the standard or sample to the wells and incubating for 90 minutes at 37°C. The liquid was then discarded, and 100μL of Biotinylated Detection Ab working solution was added to each well. The plate was incubated for 60 minutes at 37°C. Next, the plate was washed three times, and 100μL of HRP conjugate working solution was added. After 30 minutes of incubation at a temperature of 37°C, the plate is rinsed with five washes. Next, Substrate Reagent of 90μL was introduced and the plate underwent incubation for a duration of 15 minutes at 37°C. Ultimately, the addition of 50μL of Stop Solution ensued and promptly after that step, the plate was subjected to a reading at 450nm.
Histopathology: The liver tissue samples were handled with utmost care and immersed in a 10% neutral buffered formalin solution for an entire day to ensure that they were properly preserved. Before preparing the paraffin block, each specimen underwent meticulous processing. In order to gain substantial insights into the level of fibrosis and necroinflammation activity present in these tissues, proficient technicians employed Haematoxylin and eosin stains. Subsequently, using advanced software technology, researchers systematically measured the areas showcasing Fibrotic variations as well as any indications pointing towards Necroinflammation activity across six to eight different field sites from each group under examination. This rigorous methodology resulted in reliable data that enhanced our understanding of these vital physiological processes within liver cells.
Statistical Analysis: With the purpose of providing a more comprehensive understanding, it is worth noting that each experimental value was expressed as the Mean ± To undertake appropriate statistical measures, one way ANOVA analysis was performed to arrive at meaningful conclusions. The level of significance was set at probabilities of P < 0.05; P < 0.01; and P < 0.001 which were considered statistically significant and noteworthy outcomes in this study respectively.
Alanine transaminase (ALT) and Aspartate Transaminases (AST)
The findings indicate that the use of PCM damaged liver health, as evidenced by increased concentrations of AST and ALT (Figure 1). In contrast, the administration of various doses and combinations of silymarin and resveratrol resulted in different levels of hepatoprotection. Specifically, the combination with the highest dosage (SIL100+RES100) showed promising outcomes in reducing liver damage. These findings suggest that SIL and RES may have potential hepatoprotective properties; however, further analysis and experimentation are needed to draw definitive conclusions about their effectiveness.
Total Protein Level
The concentration of total protein is a crucial indicator when evaluating the condition and functioning of the liver. It is commonly utilized to determine if there are any impairments or damage to the liver. The findings imply that administering PCM led to a decrease in total protein concentration, which suggests possible dysfunction in the liver. However, when comparing with the PCM group, it was observed that treatment groups receiving combinations of SIL and RES (SIL50+RES50, SIL50+RES100, SIL100+RES100) exhibited an improvement in their total protein concentration levels. This indicates that these treatments involving SIL and RES may have acted as protective measures for maintaining proper liver function and counteracted any detrimental effects caused by PCM administration. The protective effect was comparable and dose-dependent (Figure 1).
Creatinine Level
According to the results, the research experimented with rats and measured their blood creatinine. Creatinine is a result of muscle metabolism that is eliminated from the circulation by the kidneys via filtration. Kidney injury or impairment may cause blood creatinine levels to rise. Compared to the control group, rats treated with PCM had increased blood creatinine levels (p<0.001). Creatinine levels decreased significantly in SIL+RES-treated rats (p< 0.001). As seen in Figure 2, the SIL50+RES 100 group had a significant decrease. A significant rise in creatinine levels suggests that PCM at the prescribed dose damaged the kidneys. However, this research found renal protection when PCM was given with HSCE (SIL50+RES50, SIL50+RES100, and SIL100+RES100). Compared to the PCM group, these therapy groups had lower creatinine levels. SIL50+RES100 and SIL100+RES100 demonstrated the most kidney protection potential.
Urea and Glucose Level
The study's findings showed significant differences in the blood urea and glucose levels between the rats given PCM treatment and the control group. The reported rise had a p-value that was less than 0.001 and was statistically significant. Further research revealed that when compared to rats that only received PCM therapy, animals treated with combinations of SIL50+RES100 or SIL100+RES100 successfully lowered these high urea and glucose levels (p 0.01) (Figure 2). These interesting findings provide important information on the potential synergistic interactions between various doses of SIL and RES, which might result in noticeable changes in urea and glucose levels throughout our designated experimental groups.
Lipid Level
To assess the impact of SIL+RES on fatty acid metabolism, various biochemical markers including cholesterol and triglyceride levels were examined. Prominent changes in these factors were observed. The PCM control group displayed higher levels of triglycerides and cholesterol compared to the normal control group. Notably, individuals treated with increased concentrations of SIL+RES exhibited substantial reductions in total cholesterol (119.28±4.60 mg/dL) and triglycerides (81.40±3.80 mg/dL) when compared to those who only received PCM alone (Figure 3). These effects demonstrated statistical significance as opposed to the PCM control group, highlighting that the efficacy of SIL+RES relies on dosage.
Effect of SIL+RES on Antioxidant Markers in Liver Tissue
SOD and Catalase Enzyme Activity: The experiment measured CAT and SOD levels in different groups, including a control group and groups treated with various substances. PCM decreased CAT and SOD activities, indicating oxidative stress and potential liver damage. However, combining SIL50 and RES50 increased CAT activity (865 U/g), showing potential protection against oxidative stress. Higher doses of SIL and RES (SIL50+RES100 and SIL100+RES100) further improved CAT activity (888 U/g and 917 U/g). SOD levels followed a similar pattern, with the highest in the SIL100+RES100 group (275 U/g). The increase in CAT levels indicates an enhancement in the liver's antioxidant defense system, potentially helping to counteract the oxidative stress caused by PCM. Similar to CAT, the administration of PCM resulted in a significant decrease in SOD levels, with a value of 103 U/g. This decrease further confirms the presence of oxidative stress induced by PCM. Co-administration of SIL and RES seems to protect against PCM-induced hepatotoxicity, enhancing antioxidant defenses (Figure 4).
Reduced Glutathione: The results of the experiment showed the levels of glutathione reductase (GR) activity in different treatment groups. The control group, which did not receive any treatment, had a GR activity level of 0.35 mg/g. However, when PCM was administered alone, the GR activity decreased significantly to 0.11 mg/g. This reduction in GR activity indicates a potential disruption in the antioxidant defense mechanism of the liver. In contrast, the groups treated with a combination of SIL and RES at different concentrations showed a slightly higher GR activity compared to the PCM group. The group receiving SIL50+RES50 had a GR activity of 0.14 mg/g, while the group receiving SIL50+RES100 had a GR activity of 0.19 mg/g. The highest GR activity was observed in the group treated with SIL100+RES100, which had a level of 0.26 mg/g (as shown in Figure 4). These findings suggest that the combination of silymarin and resveratrol, particularly at higher concentrations (SIL100+RES100), may have a protective effect on the liver's antioxidant defense system. The increase in GR activity in these groups indicates a potential restoration of the liver's ability to combat oxidative stress caused by PCM administration.
Nitric Oxide Level: The control group had an average NO level of 0.44 micromol/g, creating a baseline for the trial. PCM treatment increased NO levels by 1.12 micromol/g. The nitric oxide pathway was affected by PCM, resulting in enhanced NO production. In the groups receiving combinations of SIL50, RES50, SIL100, and RES100, varying effects on NO levels were observed. The SIL50+RES50 group demonstrated an average NO level of 0.85 micromol/g, indicating a partial reduction compared to the PCM group. Similarly, the groups treated with SIL50+RES100 and SIL100+RES100 displayed average NO levels of 0.8 micromol/g and 0.78 micromol/g, respectively. These results suggest a consistent reduction in NO levels when higher concentrations of SIL and RES were combined. In the groups receiving combinations of SIL50+RES50, and SIL100+RES100, varying effects on NO levels were observed. The SIL50+RES50 group demonstrated an average NO level of 0.85 micromol/g, indicating a partial reduction compared to the PCM group. Similarly, the groups treated with SIL50+RES100 and SIL100+RES100 displayed average NO levels of 0.8 micromol/g and 0.78 micromol/g, respectively. These results suggest a consistent reduction in NO levels when higher concentrations of SIL and RES were combined. A comparison between groups treated with PCM and those without showed clear statistical variations in the NO levels, as depicted in Figure 4.
IL-6 and TNF-α level: Two inflammatory indicators, IL-6 and TNF-α, were measured in the study's different therapy groups. PCM was given to the PCM group, which resulted in somewhat lower levels of IL-6 and TNF-α than the control group, which served as the baseline group. In the SIL50+RES50 group, IL-6 and TNF levels increased slightly but remained lower than in the PCM group. Similarly, the SIL50+RES100 group, combining SIL50 with a higher dose of RES100, showed slightly elevated IL-6 and TNF-α The SIL100+RES100 group exhibited slightly higher IL-6 and TNF levels compared to all other groups. Overall, the results suggest that PCM administration may suppress IL-6 and TNF-α levels, but the tested combinations did not significantly alter these markers. The impact of the combinations of IL-6 and TNF-α in PCM-induced hepatotoxicity appears limited. Further analysis is needed based on the study's objectives and hypotheses. The detailed results can be seen graphically through Figure 5.
TBARS: PCM's effects on liver oxidative stress were studied in this investigation. Different groups' TBARS levels, which indicate oxidative stress, were assessed. The control group exhibited modest TBARS levels (0.72), whereas the PCM group had considerably higher levels (1.87), suggesting enhanced oxidative stress. The SIL50+RES50 and SIL50+RES100 groups exhibited lower TBARS levels (1.11 and 0.95) compared to the PCM group. This suggests that SIL50 combined with RES50 or RES100 potentially reduced PCM-induced oxidative stress. The most significant reduction in TBARS was observed in the SIL100+RES100 group (0.82), indicating that the combination of SIL100 and RES100 was most effective in mitigating oxidative stress caused by PCM. In conclusion, the use of PCM led to a rise in MDA levels (with a significance level of p < 0.05) in rat liver tissue homogenate, as shown in Figure 6. Both SIL and RES pretreatments had comparable effectiveness in reversing the increase toward regular TBARS levels. Yet, when administered at higher dosages together (SIL+RES), they exhibited equivalent efficacy at correcting abnormal TBARS levels with an even lower significance value of p<0.001).
Hepatoprotection (%): In this study, we assessed the hepatoprotective effects of SIL+RES on liver damage. They calculated the percentage of protection provided by SIL+RES by comparing it to the negative control group, which received PCM-induced liver damage without any treatment, and the intact control group, which exhibited complete 100% hepatoprotection. The negative control group demonstrated no protection, demonstrating that PCM-induced liver damage was not alleviated without SIL+RES therapy. However, the intact control group showed 100% hepatoprotection, indicating no liver injury. To evaluate the hepatoprotection offered by SIL+RES, the researchers analyzed several biomarkers and compared their levels to those observed in the control groups. The results showed that the animals treated with SIL+RES had preserved levels of these biomarkers. This finding supports previous studies that demonstrated the efficacy of SIL+RES as a hepatoprotective agent. In conclusion, SIL+RES therapy retained liver biomarkers, indicating hepatoprotective benefits. This combination showed promise in reducing PCM-induced liver damage. SIL+RES may treat hepatotoxicity as shown in Figure 7.
Histopathology: Histopathology of liver tissue showed that SIL+RES (the combo therapy) protects the liver. PCM alone caused haemorrhage, necrosis, vacuolated cytoplasm in hepatocytes, and inflammatory cell infiltration in rats. In contrast, when a higher dose of SIL+RES was administered before PCM exposure, the liver sections demonstrated significant improvement. The damage severity was greatly reduced, and the liver tissue resembled that of the normal control group. These histopathological findings strongly support the protective effect of SIL+RES against liver injury induced by PCM and suggest its potential to mitigate the harmful effects of the toxin. These results align with previous findings from biomarker analysis and other studies, further confirming the efficacy of SIL+RES in preventing and reducing liver damage. Nonetheless, these findings highlight the therapeutic potential of SIL+RES as a hepatoprotective treatment in the context of PCM-induced liver damage (Figure 7).
To determine the effectiveness of SIL+RES in protecting against liver damage caused by PCM at varying doses, this study was conducted. PCM is a commonly used hepatotoxin in experimental cell and tissue models for inducing hepatic injury. It is typically eliminated from the body through sulfate and glucuronide conjugation. Numerous studies have established a strong link between the extent of liver damage and enzyme levels, including ALT, AST, creatinine, cholesterol as well as total protein and triglycerides. Additionally, investigations indicate that biomarkers such as catalase, SOD, and GSH are elevated due to oxidative stress caused by liver impairment. These results hence provide evidence that these indicators can be considered valid means for evaluating hepatic function [1,37]. Liver damage can be caused by N-acetyl-p-benzoquinoneimine, a harmful metabolite of PCM. This is because the liver processes PCM using cytochrome P450 monooxygenase, which generates NAPQI as a secondary metabolite. Hepatotoxicity occurs when NAPQI undergoes bioactivation via several CYP450 enzymes. It's important to comprehend these enzyme interactions when considering treatment options for potential liver damage caused by excessive dosage or prolonged use of PCM medications [38]. NAPQI, which stands for N-acetyl-p-benzoquinone imine, is normally conjugated with glutathione and excreted in urine. However, it's worth noting that GSH plays a crucial role in providing antioxidant defense to NAPQI, a toxic metabolite formed upon the consumption of PCM, which is usually conjugated with glutathione and eliminated through urine. GSH plays a crucial role in our body's antioxidant defense mechanism by fearlessly scavenging free radicals generated during metabolic processes within the liver to avoid any ensuing cellular harm. With its remarkable ability to neutralize potentially harmful substances, GSH expands boldly as an essential factor in maintaining optimal physiological functions and overall health.
Overdosage of PCM can have severe consequences on the human body due to the accumulation of NAPQI. This toxic metabolite binds irreversibly with GSH, a vital antioxidant in our body, leading to depletion and causing oxidative stress. The build-up of conjugates significantly increases reactive oxygen species production which further oxidizes GSH molecules into glutathione disulfide. Consequently, this oxidation process results in reduced levels of blood and liver GSH-a situation that makes patients more susceptible to various diseases related to low antioxidants [39-41]. When free radicals are generated in cells, Catalase gets induced. This enzyme is an antioxidant that safeguards the cell from oxidative stress caused by hydrogen peroxide. Its significant function involves shielding against lipid peroxidation's harmful impacts [40,41]. The research study revealed a significant increase in catalase levels with the administration of lower doses (50 mg/kg) of SIL+RES combination as compared to previous works. The administration of a higher dosage (100 mg/kg) showed compelling evidence of the treatment's effectiveness in normalizing catalase levels. This suggests that it may offer potential protection against tissue injury and oxidative stress attributed to the detrimental effects of free radicals. Furthermore, the findings imply that SIL+RES treatment at higher doses contributes to catalase enzyme-mediated mechanisms by restoring catalase values. The role of SIL+RES in reducing oxidative stress and its impact on tissue health is a significant finding with important scientific implications. The results of this treatment could cause glutathione levels to decrease, which can lead to major problems like acute liver necrosis and more lipid peroxidation. Decreased liver and blood GSH levels may also cause mitochondrial dysfunction. Hepatocyte death and liver steatosis may result from these processes inadvertently damaging proteins, lipids, and DNA.
Therefore, efforts must be made at every level to avert such situations. Oxidative damage biomarkers are more sensitive to obese people due to their direct association with BMI %. GSH has been identified to be responsible for preventing cell damage by scavenging free radicals produced during metabolism processes within the liver through conjugate formation that results from PCM overdosage leading ultimately to reduced levels of both blood and hepatic GHS concentration levels. The relationship between GSH depletion, hepatic damage, and the release of specific enzymes is a simple yet valuable observation in assessing liver health. It underscores the significance of measuring these enzyme biomarkers for early detection and treatment of liver abnormalities [42,43]. Hepatic parenchymal cells produce alanine transaminase, an enzyme used to identify liver damage, and are essential to liver function. Aspartate transaminase is particularly important because it detects mitochondrial abnormalities in zone 3 or the centrilobular zone of the liver. Mitochondrial distortion may induce significant hepatic damage and dysfunction, hence AST monitoring is crucial [44]. Furthermore, based on the findings from Somchit et al., it can be convincingly argued that NAPQI actively participates in the formation of protein adducts by reacting with DNA and cellular proteins. This reaction ultimately causes a cascade of negative effects such as hepatocyte dysfunction and necrosis, leading to severe liver damage. Therefore, raising awareness about preventing exposure to NAPQI is crucial for safeguarding overall health and minimizing liver injury caused due to its toxic properties [45]. Rats that are administered PCM as a hepatotoxin exhibit various histopathological abnormalities such as cellular necrosis, degenerated hepatocytes, and steatosis. Additionally, there is evidence of dilated hepatic sinusoids in affected rats. Mononuclear inflammatory cellular exudates infiltrate portal areas primarily comprising lymphocytes.
The results of this study indicate that the concurrent administration of SIL and RES may aid in the resolution of PCM-induced necro-inflammatory lesions. Shalan et al. and Shaker et al. have provided compelling evidence that SIL can reduce inflammation and modulate histopathological changes induced by CCl4, such as ballooning, necrosis, and lymphocyte infiltration in inflamed areas. Our study's findings provide support for these findings [46-48]. The investigation revealed that the combined treatments of SIL50+RES50, SIL50+RES100, and SIL100+RES100 led to enhanced liver functionality in rats in comparison to PCM administration alone. Furthermore, these treatments exhibited the ability to decrease creatinine levels, indicating a safeguarding effect on kidney function. Notably, positive alterations were observed in lipid metabolism, reflected in changes to cholesterol and triglyceride measures. The concurrent administration of both SIL and RES also resulted in strengthened antioxidant defense mechanisms, evident through elevations in catalase, superoxide dismutase, and glutathione reductase levels. Moreover, a reduction in inflammatory markers was noted, underscoring the potential therapeutic advantages. Collectively, these findings propose that the synergistic application of SIL and RES could offer a promising avenue for mitigating PCM-induced liver damage in rats.
The goal of the research was to evaluate the efficacy of silymarin and resveratrol in treating acute hepatotoxicity in rats induced by PCM treatment. The purpose of this research was to better understand the efficacy and safety of drugs used to treat hepatic dysfunction based on empirical evidence. There is a severe lack of trustworthy information available now. The research results indicate that administering different dosages of SIL and RES, such as SIL 50 + RES 50, SIL 50 +RES100, and SIL100+RES100 effectively provided remarkable protection against the hepatic toxicity induced by PCM in rats. The rats were given the combination, resulting in these findings. An evaluation of glutathione, superoxide dismutase, catalase, and thiobarbituric acid reactive compounds in the liver served to confirm the efficacy of the SIL + RES therapy. To validate the effectiveness of this treatment, total cholesterol, total protein, and triglycerides were also assessed. Alanine transaminase, aspartate aminotransferase, total protein, and total cholesterol were among the liver enzymes measured. Nevertheless, the results also emphasized the significance of dose optimization for clinical usage to maximize therapeutic benefits. In conclusion, silymarin and resveratrol reduce oxidative damage and inflammation to preserve liver function. Resveratrol and silymarin have been widely examined for their hepatoprotective benefits. They synergistically protect the liver, especially in PCM-induced hepatotoxicity. In preclinical investigations, silymarin and resveratrol show promise. They may prevent and cure liver disorders due to their antioxidant capabilities. As research proceeds, SIL+RES's potential as hepatoprotective agents becomes clearer, bringing hope for liver health and liver-related diseases. The findings expand our understanding of the possible advantages that SIL and RES offer in improving organ function, emphasizing a necessity for further investigation to establish their precise dosage requirements and general effectiveness when treating liver ailments.
• Treatment of PCM-induced hepatotoxicity Using Silymarin (SIL) and Resveratrol (RES)
• SIL and RES in dose-dependent manner reduced PCM-induced liver damage.
• SIL and RES protect kidney function through possible Reno protective characteristics.
• SIL and RES’ hepatoprotective potency through anti-inflammatory and antioxidant actions.
The authors declare no conflict of interest.
Conceptualization, Mohamed Balaha and Aftab Alam; Data curation, Ahmed A. Alamer, Ahmed M. Kabel, Rana M. Aldossari and Alhussain H. Aodah; Formal analysis, Mohamed Balaha, Ahmed A. Alamer, Rana M. Aldossari and Aftab Alam; Funding acquisition, Ahmed M. Kabel; Investigation, Mohamed Balaha, Ahmed A. Alamer, Ahmed M. Kabel, Rana M. Aldossari, Alhussain H. Aodah and Aftab Alam; Methodology, Mohamed Balaha, Ahmed M. Kabel, Rana M. Aldossari, Alhussain H. Aodah and Aftab Alam; Project administration, Mohamed Balaha and Ahmed A. Alamer; Resources, Mohamed Balaha; Software, Ahmed A. Alamer; Validation, Ahmed M. Kabel; Visualization, Alhussain H. Aodah; Writing – original draft, Mohamed Balaha, Ahmed A. Alamer, Ahmed M. Kabel and Aftab Alam; Writing – review & editing, Mohamed Balaha, Rana M. Aldossari, Alhussain H. Aodah and Aftab Alam.
This study is supported via funding from Prince sattam bin Abdulaziz University project number (PSAU/2023/R/1445).
The authors extend their appreciation to Prince sattam bin Abdulaziz University, for funding this research work through the project number (PSAU/2023/R/1445).


