Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Elisabetta Garagiola1*, Emanuela Foglia1, Federica Asperti1, Grazia Antonacci2,3, Yogini Jani4, Daniele Bellavia1 and Fabrizio Schettini1
Received: November 01, 2023; Published: November 16, 2023
*Corresponding author: Elisabetta Garagiola, LIUC Business School, LIUC- University Cattaneo, Healthcare Datascience LAB, Corso Matteotti 22, 21053 Castellanza, Varese, Italy
DOI: 10.26717/BJSTR.2023.53.008454
Objectives: Over the last few years, hospitals have embraced automated drug dispensing technologies
aiming to streamline processes, minimise medication errors, and boost safety for patients and medical
staff alike. This review endeavours to synthesise and critically assess current evidence concerning these
technologies and their integration into clinical practice.
Methods: The research, conducted in October 2022, based on two databases, Scopus and PubMed,
searches peer-reviewed articles or reviews published in English, considering a time frame between 1995
and 2022. In addition, grey literature is considered. Four distinct comparison scenarios were delineated
for evidence evaluation. Only studies using a comparative approach to describe the safety, efficacy, and
efficiency of technologies and undergoing quality assessment (Newcastle-Ottawa Scale, IMPAQHTA
model and AACODS checklist) were incorporated. Results were synthesised with a narrative approach.
Results: After removing duplicates, 203 papers were screened, and nine observational studies were
included in the narrative synthesis. Evidence indicates that automation substantially mitigates errors
in drug administration, encompassing dosage mistakes and curtails errors in drug dispensing and
distribution. Furthermore, it economises the time healthcare professionals devote to medication
management.
Conclusions: Automated dispensing technologies bolster safety and efficiency for both patients and
healthcare staff. Yet, existing evidence chiefly pertains to either pharmacy-based or ward-based scenarios,
side-lining integrated solutions across both and results are not completely generalizable considering
the observational local approach. To truly gauge these technologies’ merits, a broad multi-dimensional
research lens is essential, furnishing insights for informed decision-making.
Keywords: Automated Technologies; Hospital; Drug Management; Medication Errors
The pivotal role of medication administration in patient therapy is undeniable, acting as a fundamental pillar for ensuring safety and well-being, particularly considering that a significant majority of hospitalised patients need pharmacological intervention as part of their healthcare regimen [1]. The multifaceted landscape of the medication management process within the hospital milieu encapsulates a spectrum of activities, including the prescribing, preparation, and dispensing phases, the subsequent administration to the patients, and vigilant observation of effectiveness and safety, notably potential adverse events or side effects [2]. Drug administration frequently represents the culmination of the drug management process, and consequently, errors in this phase could significantly compromise patient safety [3], potentially prolonging hospital stays and escalating healthcare expenditure due to systemic inefficiencies or failures [4]. However, quantifying the impact of errors in clinical practice, including potential harm throughout the drug management process, often poses a challenge, primarily because some errors may not result in harm or are relatively minor. On the other hand, some errors, while corrected before precipitating adverse events, could nonetheless create additional workload for healthcare professionals and organisations [5]. In many hospitals drugs are still stored in an alphabetical order on open shelves.
This system is susceptible to errors, given the human propensity for mistakes, particularly in high-stress or complex situations, during communication breakdowns, or in the absence of standardisation [5- 7]. Manual dispensing often involves ward nurses and pharmacists in tasks that could be automated, thereby enabling them to divert more time to value-added clinical activities [8]. Additionally, the literature suggests that many administration errors remain undetected and previous studies indicated a possible role for automation and related technologies in mitigating this issue [9]. The advent of automated logistic solutions for drug management in hospital settings has garnered increasing attention in recent years [10], considering these solutions as potential instruments to rationalise processes, reduce medication errors, and enhance patient safety [11,12]. Automated logistic solutions comprise diverse technologies and systems designed to automate various stages of drug management, including ordering, dispensing, and administering medications [13], due also to their nature of information technologies [14]. Examples include computerised physician order entry (CPOE), barcode medications administration (BCMA) systems [15], pharmacy dispensing technologies [16], and dispensing cabinets. Such automated systems could mitigate the risk of human errors, reducing medication errors and improving patient safety [17], alleviating the workload on healthcare professionals.
Previous studies suggest that augmenting the degree of automation within one or more stages of the medication management process could reduce error rates [3-18]. As for the efficiency profile of automated dispensing systems, some authors have demonstrated that these logistics systems may confer advantages to healthcare professionals and facilities at large, potentially saving time for hospital pharmacies, departments, and nursing staff, improving medication management processes, and reducing the time required for medication administration [19]. Automation has been particularly lauded in the dispensing stage, where medication errors (MEs) occur [20], and traditional manual dispensing systems are more prone to mistakes and inefficiencies [21]. However, a comprehensive analysis of the supplementary benefits and advantages resulting from implementing integrated solutions, both in the centralised pharmacies and wards, remains limited.
This review was undertaken to identify, summarise, and critically assess the current state of knowledge and available evidence about automated solutions for drug dispensing processes within hospital settings and their integration within the broader drug management and administration process [22]. The objective was to assess the safety, efficacy, and efficiency of various automation scenarios within hospital dispensing processes. This endeavour is expected to offer insights into distinct and additional benefits of existing automated solutions for hospital dispensing, thereby assisting the decision-making process of healthcare managers and other hospital stakeholders. Moreover, the findings of this study are anticipated to augment the understanding of the subject matter and lay the groundwork for the design of more comprehensive empirical investigations. These could enhance current hospital practices in drug management and administration processes by identifying existing knowledge gaps that could inform the implementation of such technologies.
A systematic literature review with a narrative synthesis [23] was conducted in October 2022 to gather evidence about safety, efficacy, and efficiency profiles concerning four scenarios.
• Scenario 1, related to the drug manual management and dispensing
(see also [8]).
• Scenario 2, in which centralised pharmacy automated dispensing
systems are implemented (described in [16]).
• Scenario 3, in which decentralized systems and dispensing
cabinets are implemented in the wards, to help drug traceability
and dispensing activities (defined in [24]).
• Scenario 4, in which all the technologies of Scenarios 2 and
3 are integrated into a hybrid automated system (see also
[25]).
PRISMA methodology [26] was used to conduct the systematic review. The PICO approach (Patient/Population, Intervention, Comparator and Outcome) was used to define the research questions and shape the review [27,28] was as follows.
• Patient/population: drugs dispensed in the hospital setting
(in-patient and out-patient).
• Intervention: automation in the drug distribution system (in
the Pharmacy and/or in the Wards settings that are represented
by Scenarios 2, 3, and 4).
• Comparator: the absence of automated distribution systems
(represented by Scenario 1).
• Outcome:
(i) Safety considering all the potential errors throughout the
drug management process (outpatient dispensing error rate, at
discharge dispensing error rate, labelling dispensing error rate,
documentation dispensing error rate, medication administration
error, dosage error rate, etc…);
(ii) Efficacy referring to the ability of an automated system to
curtail the dispensing error rates;
(iii) Efficiency is related to the reduction of the drug preparation
time and drug administration time.
Two separate databases, Pubmed and Scopus, were explored to find peer-reviewed articles with a specific set of keywords present in either the title or abstract. These keywords included: “Drug*” or “Medicine” or “Medication”, “Centralised” or “Pharmacy Based”, “Cabinet” or “Ward Based”, “Automated Dispensing System” or “Robot” or “Device” or “Machine”, and “Safety” or “Efficacy” or “Efficiency”. In addition, also the grey literature was investigated. The selection of these keywords followed a structured process of ideation inspired by Osborn’s “brainstorming” model [29]. This process was conducted by a team of eight healthcare professionals with extensive experience in hospital settings to ensure a comprehensive and relevant search. Then the team performed a prioritization process to determine the most pertinent keywords to be incorporated into the search strategy. Moreover, publications were also identified through hand-searching and cross-referencing citations. Evidence between 1995 and 2022 was considered in the search process, ensuring a comprehensive exploration of the topic, acknowledging the historical antecedents, and enabling a more thorough analysis of the development over time, considering also the first evidence related to the manual scenario.
Study Inclusion and Exclusion Criteria
Studies were included if they met the following criteria:
(i) Peer-reviewed journal articles, reviews and grey literature
published in English between 1995 and 2022;
(ii) They specifically assessed the safety, efficacy, and efficiency
of automated dispensing technologies within a hospital setting;
and
(iii) Comparative studies involving at least two of the four scenarios
under evaluation.
Exclusion criteria encompassed articles in languages other than English and publication types such as conference proceedings, book chapter and discussion papers. Moreover, any article demonstrating substantial flaws in its quality was also excluded. Studies solely focusing on automated technologies like prescribing systems and Barcode Medication Administration (BCMA) were not incorporated into the narrative synthesis.
Screening And Selection Process
One author performed the initial database search, exporting citations into a reference management software (Mendeley Ltd). This software facilitated the process of duplicate removal, which was supplemented by a manual check to eliminate any overlooked redundancies. A double-blind review process was subsequently conducted. The selection process was initiated by two authors — a health economist and a management engineer, both with expertise in the healthcare sector. They screened the titles and abstracts of the citations and then reviewed the full texts to identify pertinent papers. A further review of the selected papers was conducted by two other authors, specialists in health technology assessment and pharmaceutical logistics, to verify their eligibility for inclusion in the review. In the event of discrepancies, issues were resolved through collaborative discussion until a consensus was reached among the authors. The validated articles were then forwarded to the next stage for quality assessment and data extraction.
Study Quality Assessment
The quality and potential risk of bias in the selected studies were evaluated by an expert panel of eight professionals, encompassing pharmacists, nurses, decision-makers, and other specialists such as biomedical engineers and management engineers. Their evaluations employed both qualitative and quantitative approaches, aiming to meticulously assess the technologies under investigation. From a qualitative standpoint, the Newcastle-Ottawa Scale [30] was used for the assessment of observational studies, evaluating the three following aspects:
(i) The selection of the study groups,
(ii) The comparability of the groups and
(iii) The ascertainment of either the exposure or outcome of interest
for case-control or cohort studies.
On the quantitative front, the panel adopted the evaluation tool proposed by the IMPAQHTA model [31]. This model employs a numerical rating system, ranging from 1 (insufficient) to 4 (excellent), to gauge the following aspects:
(i) Overall quality of the paper,
(ii) Generalizability of the results, and
(iii) Completeness of the findings. The grey literature was evaluated
according to the AACODS checklist, including six different
domains: Authority, Accuracy, Coverage, Objectivity, Date and
Significance [32]. More detailed information can be found in the
Supplementary material.
In line with the aim and objective of this review, a data extraction template was created and incorporated into an Excel spreadsheet. The extraction process was carried out by two authors, with any discrepancies resolved through deliberation among the other authors until consensus was achieved. The data extracted from each article included general identifying information (authors, title, publication year, source title, affiliations, abstract, authors keywords), methodological aspects (study design, information about data collection and analysis) and a range of items related to the three primary outcomes of interest considered for the review process (safety, efficacy, efficiency). The comprehensive data extraction template can be found in the Supplementary material.
Data Synthesis and Presentation
A bibliometric network was created considering the information regarding the papers included in the review to find the most relevant subjects within those scientific publications, which would become the focus of this research, helping identify possible knowledge and literature gaps. A bibliometric network consists of nodes and edges used to determine the correlation between the words and the strength of the connection through the edges [33]. To help create and visualise the bibliometric network, the software VOS Viewer was used to perform a distance-based network, in which the distance between two nodes indicates their correlation and the bigger the node, the bigger the number of citations the word has [32]. Then, given the anticipated heterogeneity in methodology and resulting data [34], a narrative synthesis supplemented by tabular representation was selected for this review. Findings about the safety, efficacy, and efficiency profiles were summarised for the four scenarios under examination. The analysis only considered primary outcomes as delineated in the PICO strategy. Where available, data reflecting statistical significance between different methods were reported. Microsoft Excel, provided by Microsoft Corporation, served as the primary tool for data analysis and presentation.
The PRISMA flowchart of the review is reported in Figure 1. Nine peer-reviewed publications [35-43] out of the 261 records retrieved with the initial search, met the eligibility criteria Table 1. The evaluation of the scientific evidence indicated a potentially high risk of bias, and a low-quality literature level was observed for all three outcomes of interest Table 2. Given the observational nature of included studies, the results presented might not be easily replicable, and diverge in different settings. This factor contributed to the lower scores in the IMPAQHTA model regarding replicability Table 2. In addition, the retrieved biases affect the results, which could not be used as unique evidence for the decision-making process and to perform a complete evaluation of drug automated dispensing systems for the hospital setting, highlighting the need to also collect further evidence such as expert opinion and perceptions, or design further studies to cover the knowledge gaps. Figure 2 shows the results of the defined bibliometric network, performed using keywords identified by the authors in their papers, clearly generating three clusters of reference. The first keywords, the ones represented with the green colour, are related to the departmental problems, which then resulted connected with the institutional part of centralised pharmacy. The third aspect that was considered by the already published scientific evidence is related to the results or outcomes. The outcomes resulted studied with a single perspective approach, not allowing an integration between the decentralised and the centralised levels. Findings concerning the three different dimensions of outcomes under analysis are described below.
Table 2: Risk of bias evaluated with the Newcastle-Ottawa Scale and final synthetic result from the assessing tool suggested by the IMPAQHTA model, considering Efficacy/Safety and Efficiency as Outcomes.
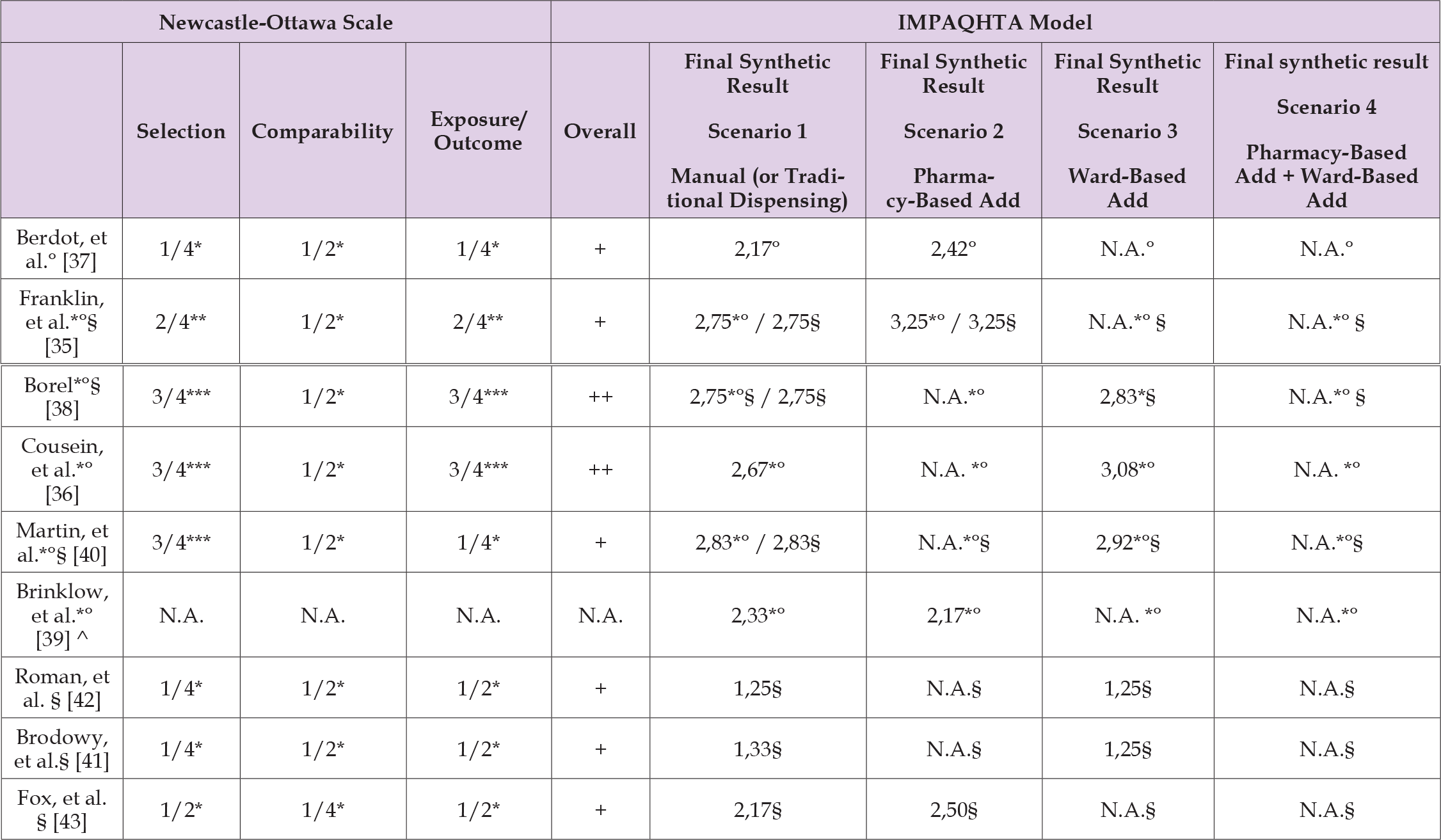
Note: *Considering Safety as an Outcome
°Considering Efficacy as an Outcome
§Considering Efficiency as an Outcome
N.A. if there is no evidence for the Scenario under assessment and so the Quality Assessment was not applicable or if the validation model could not be
applied considering the nature of the publication type
^This study, using the AACODS checklist was validated with a positive score of 28/34 (82.35%).
Two of the selected studies performed a comparative analysis of safety aspects across various scenarios Table 3 [35,36]. However, [35] were unable to test the difference between scenarios due to the small sample size, whereas [36] found statistically significant difference between the results from the various scenarios only for one of the three metrics assessed. [35] revealed that automation in the Central Pharmacy could lead to a substantial reduction in dispensing errors for outpatients and in labelling and documentation errors within the Central Pharmacy, compared to traditional dispensing. However, these differences were not calculated considering that the data were available for less than 50% of the prescriptions assessed during the study. On the other hand, [36] compared manual dispensing with a ward-based automated scenario, discovering that automated systems implemented in the wards led to statistically significant improvements in terms of reducing Medication Administration Errors (MAEs), dosage errors, and drug error rates (p<0.05).
Four of the selected papers examined the efficacy dimension and conducted comparisons among various scenarios Table 3. All the studies utilized the dispensing error rate as a measure of the dispensing process efficacy. They reported that scenarios employing automated dispensing technologies, either in the Pharmacy or in the Wards, demonstrated statistically significant lower error rates (p<0.005) compared to the scenario involving manual dispensing. Two studies, [35,37] compared Pharmacy-based automated systems with manual dispensing. Their findings suggested that the implementation of automation in the Central Pharmacy resulted in a reduction of dispensing errors — from 2.9% to 1.7% (p<0.001) and from a range of 1.4%- 2.7% to 0.6%-1% (p<0.005), respectively. Another study conducted by [38] determined that dispensing errors significantly decreased when automation was introduced in the Wards (comparison between Scenario 3 and Scenario 1) (p<0.005).
Six studies delved into the efficiency dimension from various perspectives among the drug management process (Table 4). Two studies juxtaposed the Central Pharmacy-Based automated scenario with the manual dispensing scenario [35] reported that pharmacy automation could enhance the time allocated for picking and the total turnaround time for discharge (p<0.01). [43] examined the efficiency outcomes before and after implementing a central dispensing drug system, demonstrating a reduction in the overall time for drug dispensing by 15%, attributable to the introduction of automation. Four studies contrasted the Ward-based automated scenario with traditional dispensing. [38] found that Ward-based automated systems could reduce the time spent on administration (p=0.003). Conversely [42], reported that ward automation could potentially increase the average medication retrieval time, thereby negatively impacting the efficiency dimension, considering the entire medication management process. This phenomenon could be explained by healthcare professionals’ queuing considering that only one person could use an ADD system at any time [42].
The present review identified nine studies that assessed safety, efficacy, or efficiency of the four comparative scenarios, depending on the technologies’ introduction. The evidence suggests the existence of ADD systems’ benefits; however, none of the studies has detailed the incremental benefits of centralised versus decentralised systems, nor have they exhaustively explored the impact of automation on efficacy, particularly concerning process time. In addition, other errors, such as prescribing errors, should persist if organisations do not adopt integrated systems, even with the introduction of an electronic prescription system [44]. Additionally, specific risks and disadvantages have been associated with using automated dispensing technologies. As per the literature, implementing automated technologies can introduce new organisational and technical risks due to alterations in the dispensing process and the organisation of hospital tasks [45,46] proposes that changes in the work routine following the introduction of automated dispensing technologies can be managed by organising regular meetings with professionals involved in the dispensing process. This would foster knowledge sharing, heighten awareness of automated solutions, and facilitate revising human resource plans in the early stages of implementation. This approach could impact organisational investments related to the learning curve, an impact that warrants further investigation.
This systematic review is a first attempt to present a comparative analysis of various levels of automation in drug dispensing systems within a hospital setting, synthesising existing research findings from a broad spectrum of studies and providing a comprehensive understanding of the current state of knowledge on the topic. From a practical standpoint, this study indicates that ADD systems could enhance drug dispensing processes’ safety, efficacy, and efficiency profiles in hospitals. Consequently, these technologies provide an opportunity for healthcare facilities to optimise their operations, reallocate resources more effectively, and augment patient safety. On a theoretical level, this study elucidates the technical profile of these automated drug dispensing systems from a multi-dimensional perspective. However, the diversity in methodologies, measures, quantitative approaches and investigated primary outcomes poses several challenges in making direct comparisons. Another limitation concerns the efficacy profile related to the implementation of technological solutions. Existing literature only presents data for Scenarios 2 and 3. In contrast, efficacy data for Scenario 4 needs to be more studied, hindering comparisons between scenarios and obscuring the incremental benefits of introducing integrated drug management systems between the Central Pharmacy and the Wards. It is also worth noting that factors such as hospital size or the number of human resources involved in the drug dispensing process could have influenced the results.
Finally, considering the quality assessment and the observational and monocentric nature of the evidence, the findings may not easily be replicable or extendable to other organisational settings. In contrast, considering all the potential advantages and benefits for healthcare facilities and patients, more comparable evidence of centralised, decentralised, and integrated systems should be produced, also guiding the training and allocation of resources within the hospital setting. The present review identified different literature gaps that must be evaluated and investigated deeply. Firstly, some dimensions were not assessed in the literature, and future research efforts could be directed towards integrating this literature review into a multi-dimensional assessment that not only considers safety and efficacy but also embraces other aspects, such as organisational, and social implications, also involving a panel of healthcare professionals (including pharmacists, nurses, decision makers, IT professionals, and biomedical engineers) from different countries, to enhance results’ validation and improvement, but also the generalizability of the results. In addition, the incremental benefits related to the integration of multiple automated systems, joining drug dispensing solutions in conjunction with other systems (e.g., BCMA or electronic prescribing) and extending the analyses to the entire logistics process from the central pharmacy to the wards, considering other drug process management phases (e.g., drug preparation, drug labelling, documentation preparation), must be supported by the need for more evidence-based information, opening up avenues for future research, both from a clinical and a managerial perspective.
In conclusion, also to support the appraisal phase and the decision- making process, future advancements and research should extend to incorporate economic considerations. This would facilitate a more nuanced understanding of economic sustainability linked to the deployment of automated solutions, an aspect often highlighted by healthcare professionals as a hurdle to their broader adoption.
Author Contributions
EF conceived the study, participated in its design and coordination, and helped in drafting of the manuscript; EG, FA, DB and FS performed the literature search, the data extraction and the data synthesis; FA and EG drafted the manuscript; EF, YG and GA critically revised the work. All authors read and approved the final manuscript.
Funding
One author is funded by the National Institute for Health and Care Research Applied Research Collaboration Northwest London. The views expressed in this publication are those of the authors and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care.
EG, EF, FA, GA, DB, FS were funded by BD, being supported with research grants or fee for educational activities, but this review was conducted independently.
Competing Interests
The authors of this review do not have any conflicts of interest. The review was not registered and the ethical approval was obtained by the Carlo Cattaneo – LIUC University Ethical Committee (protocol number #R05-23, dated February 24th, 2023).


