Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Jinhua Luo1,2, Linlin Yang2, Tuming Zhang2, Yueying Wu2 and Yu Yang2*
Received: October 14, 2023; Published: November 09, 2023
*Corresponding author: Yu Yang, Department of Geriatrics, Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong Province, China
DOI: 10.26717/BJSTR.2023.53.008436
The study aimed to explore the relationship between relative grip strength and the development of arterial stiffness. The final analysis included 6,033 people aged 45 years or older who had participated in both the 2011 and 2015 waves of the China Health and Retirement Longitudinal Study surveys. The grip meter was used to measure grip strength, and relative grip strength was determined by dividing grip strength by body mass index. A validated mathematical equation was used to calculate estimated pulse wave velocity (ePWV). ePWV ≥10 was defined as arterial stiffness. The results showed that the incidence of arterial stiffness in this population was 23.2%. After adjusting for related factors including age, educational level, marital status, history of smoking, history of alcohol consumption, history of diabetes and history of hyperlipidaemia, relative grip strength was associated with the development of arterial stiffness negatively. The OR (95% CI) was 0.67 (0.52~0.87) for men and 0.61 (0.44~0.85) for women. Stratified analysis suggested that the relationship between relative grip strength and the development of arterial stiffness remained consistent in different subgroups. The study found that the occurrence of arterial stiffness is linked to relative grip strength, and interventions to improve relative grip strength may benefit arterial health.
Keywords: Muscle Strength; Arterial Stiffness; Middle-Aged and Older Population; Longitudinal Study
Abbreviations: CVD: Cardio Vascular Disease; BMI: Body Mass Index; CHARLS: China Health and Retirement Longitudinal Study; ePWV: Estimated Pulse Wave Velocity; IQR: Interquartile; cfPWV: Carotid-Femoral Pulse Wave Velocity; SD: Standard Deviation
Cardio Vascular Disease (CVD) is a major contributor to the global burden of disease, requiring immediate attention for prevention and management in public health worldwide. The presence of arterial stiffness increases the risk of CVD and is predictive of future adverse cardiovascular events and all-cause mortality in the general population [1]. It is therefore beneficial to identify and prevent risk factors related to arterial stiffness. Sarcopenia is the loss in muscle strength as well as muscle mass that occurs as muscle tissue ages [2]. Studies indicate that individuals with sarcopenia are more likely to have arterial stiffness than those without [3,4], suggesting a link between deterioration in muscle tissue quality and arterial stiffness. Muscle strength, which is considered by the latest guidelines as a core indicator of sarcopenia [2,5], also deserves attention because of its association with arterial stiffness and because it is more readily available and applicable for risk screening, disease prediction, and management. Muscle strength is often assessed in clinical settings using grip strength, although Recent research has been inconclusive when it comes to the link between grip strength and arterial stiffness [6-9]. Irrespective of gender, there is a positive correlation between grip strength and Body Mass Index (BMI) in middle-aged and older populations [10].
On the other hand, the effect of BMI was not taken into account in any of the above studies, and their limited sample sizes may have played a role in the inconclusive results. We speculate that relative grip strength, which adjusts for the effect of body size on muscle strength, specifically BMI-corrected grip strength, may provide a more accurate indication of the true relationship between muscle characteristics and arterial stiffness. Therefore, this research used data from the China Health and Retirement Longitudinal Study (CHARLS) to analyze the relationship between relative hand strength and the likelihood of developing arterial stiffness.
Study Design and Participants
CHARLS aims to examine the current state of aging in China by utilizing well-collected data from Chinese households and individuals aged 45 years and older. The CHARLS baseline survey was conducted in 2011 using a multistage proportional to size sampling design, and three follow-up surveys were conducted in 2013, 2015 and 2018 [11-13]. Data for this study were obtained from the initial survey conducted in 2011, which included 17,707 participants, and the follow-up survey conducted in 2015. A total of 11,674 participants were disqualified. The reasons were as follows:
1. Insufficient information on weight, height, grip strength, age, or age less than 45 years;
2. Incomplete data on hyperlipidemia history, diabetes history smoking habits, or history of alcohol consumption;
3. Individuals meeting the criteria for a diagnosis of arterial stiffness in 2011 or lacking complete follow-up information in 2015.
Assessment of Relative Grip Strength
Participants were instructed to exert maximum force while squeezing the dynamometer (TMWL-1000, Yuejian, China) in order to assess the strength of their hands, measured in kilograms. The maximum value is the result of two tests on the dynamometer with both hands at right angles. Relative grip strength was calculated by dividing hand grip strength by BMI, which is obtained by dividing weight by height squared.
Measurement of Arterial Stiffness
The equation described by Greve SV, et al. [14] was used to calculate the Estimated Pulse Wave Velocity (ePWV). The ePWV value was obtained by substituting the values of age and mean blood pressure into the equation: ePWV = 9.587 - 0.402 × age + 4.560 × 10-3 × age2 -2.621 × 10-5 × age2 × mean blood pressure + 3.176 × 10-3 × age × mean blood pressure - 1.832 × 10-2 × mean blood pressure. Mean blood pressure was calculated as diastolic blood pressure + 0.4 × (systolic blood pressure - diastolic blood pressure) and the unit of ePWV is m/s. Following previous literature [15], ePWV≥10 m/s was defined as arterial stiffness.
Potential Covariates
The effects of socio-demographic characteristics and health-related factors were considered as covariates in the analysis, with reference to related published studies [6-9]. The sociodemographic factors considered were age, sex, educational level, and marital status. Health-related factors consisted of smoking history (no/yes), history of alcohol consumption (no/yes), history of diabetes(no/yes), and history of hyperlipidemia(no/yes).
Statistical Analysis
The statistical software package R (http//www.R-project.org) and Free Statistics software version 1.8 (http//www.clinicalscientists.cn/freestatistics) were employed for conducting all analyses. Quantitative data with a normal distribution were expressed as mean ± standard deviation, while skewed distribution data were expressed as median (Interquartile, IQR). Group comparisons were performed using analysis of variance and Kruskal-Wallis rank sum test. Qualitative data were presented as percentages and compared using the chi-squared test. The regression analyses encompassed unadjusted and adjusted models, wherein adjustments were made for covariates including age, education, marital status, smoking history, alcohol consumption history, diabetes, and hyperlipidemia. A significance level of less than 0.05 was employed to determine statistical significance.
Participants, Characteristics at Baseline
There were 2733 males (47.9%) and 3300 females (52.1%) among the participants. The cumulative prevalence of arterial stiffness in the entire population was 23.2%, with 27.3% in males and 24.6% in females. In comparison to the control group, the arterial stiffness group exhibited a more advanced age, a greater proportion of male participants, a higher percentage of individuals with an educational attainment below lower secondary level, and a higher prevalence of alcohol consumption history, smoking history, and diabetes history. In addition, the arterial stiffness group had reduced grip strength, reduced relative grip strength and increased ePWV at baseline. The distribution of marital status, hyperlipidemia and BMI was basically the same in the control and arterial stiffness groups (Table 1).
Note: IQR, Interquartile Range; SD, Standard Deviation; BMI: Body Mass Index; RGS: Relative Grip Strength, Ratio of Grip Strength and BMI; ePWV: estimated Pulse Wave Velocity
Association Between Relative Muscle Strength and Incident Arterial Stiffness
Due to significant differences in grip strength between the sexes, separate analyses were performed for men and women. When not adjusted for covariates, there was a negative association between relative grip strength and incident arterial stiffness in men [OR (95% CI): 0.37 (0.29~0.46)] and women [OR (95% CI): 0.37 (0.28~0.5)]. After controlling for variables including age, educational attainment, marital status, smoking history, alcohol consumption history, diabetes, and hyperlipidemia, the negative correlation between relative grip strength and the occurrence of arterial stiffness persisted [OR (95% CI): 0.67 (0.52~0.87) for males and 0.61 (0.44~0.85) for females]. According to the trichotomies, the grip strength of each sex was categorised into three groups: low, medium and high. After adjustment for the aforementioned covariates, the high relative grip strength group [(OR (95% CI): 0.67 (0.52~0.85) for men and 0.74 (0.57~0. 95)] for women) showed a lower risk of arterial stiffness, while the medium relative grip strength group [(OR (95% CI): 0.91 (0.72~1.14) for men and 1.13 (0.89~1.42)] for women) showed a similar risk compared with the low relative grip strength group (Tables 2 & 3) Stratified analysis revealed that the association between relative grip strength and the progression of arterial stiffness remained uniform across various subgroups., including different age groups, marital status, history of alcohol consumption, history of smoking, history of diabetes mellitus and history of hyperlipidemia, with on interaction effect observed (Figures 1 & 2).
Note: Model 1: not adjusted; Model 2: adjusted for age, education, marital status, history of smoking, history of drinking alcohol, history of diabetes and history of hyperlipidemia.
Note: Model 1: not adjusted; Model 2: adjusted for age, education, marital status, history of smoking, history of drinking alcohol, history of diabetes and history of hyperlipidemia.
The evaluation of aortic stiffness is often based on Carotid-Femoral Pulse Wave Velocity (cfPWV), which is widely recognized as a standard reference [16,17]. However, it requires specific machine to measure, which limits its use in large-scale epidemiological studies. A mathematical equation is used to calculate ePWV [14,18], which is strongly correlated with cfPWV [14], predicts vascular aging [19], and is also linked to cardiovascular disease prognosis [20] and all-cause mortality [21]. Comparison with cfPWV, ePWV demonstrates a stronger correlation with objective markers associated with arterial stiffness like carotid intima-media thickness, and is considered a viable surrogate for cfPWV in assessing arterial stiffness in large population studies [15]. In this large population study, we used ePWV as a measure of arterial stiffness and found that the prevalence of arterial stiffness among the Chinese middle-aged and senior citizens was 23.2%. The study findings indicate a significant association between relative grip strength and the development of arterial stiffness in both male and female populations, even after adjusting for factors such as age, education, marital status, history of smoking, history of alcohol consumption, diabetes and hyperlipidemia. The high relative grip strength group in both male and female groups experienced a 33% and 26% decrease in the likelihood of developing arterial stiffness compared to the low relative grip strength group. Relative grip strength exhibited a consistent and inverse correlation with the development of arterial stiffness in various age groups, regardless of gender, marital status, alcohol consumption history, smoking habits, diabetes history, or hyperlipidemia history.
As far as we know, this study is the initial investigation into the association between BMI-corrected grip strength and the progression of arterial stiffness. Relative grip strength is determined by comparing absolute grip strength to BMI, primarily to account for the influence of body size on grip strength. However, the correlation between absolute grip strength and arterial stiffness is inconsistent [6-9]. A study conducted in an elderly population living in a Japanese community found that there was an independent correlation between grip strength and arterial stiffness [9]. Nevertheless, it is imperative to acknowledge that the study employed a limited sample size and adopted a cross-sectional research design. In contrast, a study by S.C. VAN DIJK et al. suggested that there was no significant correlation observed between grip strength and the probability of encountering arterial stiffness among older individuals [6]. According to our analysis, while absolute grip strength was found to be correlated with the prevalence of arterial stiffness, it was not found to be correlated with the onset of arterial stiffness independently (Supplementary Table 1). On the other hand, BMI-adjusted relative grip strength demonstrated a consistent association with the development of arterial stiffness, indicating its potential to enhance comprehension of the connection between alterations in muscle tissue and the occurrence of arterial stiffness. Therefore, it has the potential to be used in research focused on the prevention of arterial stiffness. Research has also shown that there are gender differences in the correlation between grip strength and arterial stiffness, with a stronger correlation observed in men compared to women [7].
Table 4: Supplementary Table 1: Association between grip strength and arterial stiffness in cross-sectional and longitudinal analysis.
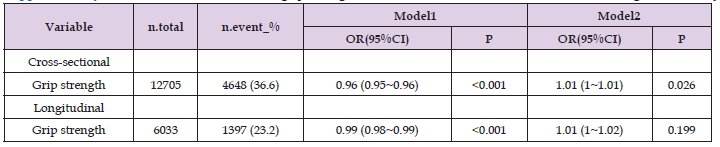
Note: Model 1: not adjusted; Model 2: adjusted for age, gender, education, marital status, history of smoking, history of drinking alcohol, history of diabetes and history of hyperlipidemia.
However, in the current study, the odds ratios when examining the correlation between relative grip strength and progression of arterial stiffness were similar in both the male and female populations, as well as in their subcategories with different characteristics. This implies that there is no disparity between genders concerning the link between relative grip strength and arterial stiffness. The association between arterial stiffness and muscle strength parameters, such as relative grip strength, is not well understood. However, some studies have provided important insights. With ageing, muscle tissue undergoes significant changes, such as degeneration of muscle fat and changes in muscle fiber properties, which can lead to a decrease in muscle strength [22]. This process is accompanied by increased chronic inflammation [23], oxidative stress [24], insulin resistance [25,26], and other changes at the molecular level, all of which have the potential to precipitate the onset of arterial stiffness. Additionally, age-related changes in the levels of sex hormones [27,28], growth hormone [29,30] and other hormones due to ageing are closely associated with a decline in muscle quality and vascular ageing. It is worth mentioning that decreased muscle strength and arterial stiffness have similar risk factors, including inadequate insufficient physical activity [31], poor nutrition status [32,33], and abnormal glucolipid metabolism [34-37]. These factors could potentially explain the association, but further basic medical and experimental research is needed to confirm this.
The current study has several notable advantages. Firstly, it includes a nationally representative sample with a substantial number of included populations, ensuring excellent data quality. In addition, the academic community has recognised and published many papers of high quality in this area. Secondly, this study stands as the pioneering research investigating the longitudinal correlation between relative grip strength and arterial stiffness. However, this study still has the following shortcomings: First, although we adjusted for many common confounders, there are still genetic factors and exercise levels that were not adjusted for; Second, arterial stiffness was calculated and defined by a validated formula rather than the gold standard (cfPWV), but the consistency of the above formula with the gold standard has also been validated [15] and theoretically does not affect the final results in a large sample study; Finally, the study included a Chinese community-based population and extrapolation to other populations requires caution, especially considering the apparent ethnic differencesin muscle quality [38].
The development of arterial stiffness is linked to relative grip strength, and people with low relative grip strength are at a heightened risk of developing arterial stiffness. Consequently, enhancing the screening and management of arterial stiffness becomes imperative to mitigate the likelihood of cardiovascular disease among those with diminished relative grip strength.
We express our gratitude to all the participants for their valuable contributions to the China Health and Retirement Longitudinal Study.
The authors declare no financial interests or conflicts of interest.


