Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Amy Wong Kie Ting1, Nabila Perveen1, Syed Atif Raza2 and Naeem Hasan Khan1*
Received: October 03, 2023; Published: October 17, 2023
*Corresponding author: Naeem Hasan Khan, Faculty of Pharmacy, AIMST University, Bedong, Kedah DA, Malaysia
DOI: 10.26717/BJSTR.2023.53.008386
Background: To evaluate the percentage purity of different brands of amlodipine tablets available in local market by using UV-Spectrophotometric method and structural characterization through FTIR Spectroscopy.
Objective(s): Quantitative determination of different brands of amlodipine tablets available in Malaysia by using UV spectrophotometric and Fourier transform infrared red spectroscopy method. To provide simple and cost-effective spectrophotometric method for quantitative analysis.
Material And Methods: For UV-spectrophotometric method, the amlodipine sample was treated with methanol as a solvent. For structural characterization method, amlodipine powdered sample was placed onto the crystal plate and scanned by using spectrum software. Disintegration tester was used for amlodipine tablets. The temperature of the distilled water was maintained at 37˚C (±1˚C).
Results and Discussion: For UV-Spectrophotometric method, the samples D and E had passed the limit specified by British Pharmacopoeia (B.P), whereas sample D, E, F, G and H had passed the limit specified by United State Pharmacopeia (U.S.P). For structural characterization, the functional groups of alkynes, alkanes, alkenes and organic halides were present in all the amlodipine samples with the respective wavelength number. A total of eight brands of amlodipine tablets were used in the research. All the different brands of amlodipine tablets were disintegrated within15 minutes. This indicated that the amlodipine tablets can be rapidly absorbed through oral route.
Conclusion: The brand D was the best pure, while brand A showed poor purity of drug among all other eight brands of Amlodipine tablets. The assay of the pharmaceutical products should be always performed to ensure the product was maintained in safety, quality, quantity and efficacy condition. All the brands of amlodipine tablets with a temperature of 37˚C (±1˚C) disintegrated within 15 minutes and results showed that the orally taken drug can be easily disintegrated into human body.
It contains a dihydro-pyridine, a member of monochlorobenzene, an ethyl ester, a methyl ester and a primary amino compound. IUPAC Name (RS)-3-ethyl 5-methyl 2-[(2-aminoethoxy) methyl]-4(2-chlorophenyl)-6-methyl-1,4- dihydropyridine-3,5-dicarboxylate. Amlodipine may be used alone or in combination with other antihypertensive or antianginal agents for the treatment of: hypertension, coronary artery disease, variant angina, chronic stable angina. Tablets containing 2.5mg, 5mg and 10mg are available. Amlodipine is a calcium ion antagonist or slow-channel blocker which can inhibit the influx of Ca2+ ions into both cardiac muscle and smooth muscle. Amlodipine will bind to both dihydropyridine and non-dihydropyridine sites in order to cause the contraction of cardiac muscle and vascular smooth muscle. Both contractions are dependent on the movement of extracellular Ca2+ ions into the cells by the ion channels. Amlodipine will selectively block the Ca2+ ion influx across the cell membrane. Thus, amlodipine will exert direct actions and effects on vascular smooth muscle [1-4].
Amlodipine is approximately 90% converted to inactive metabolites via hepatic breakdown with 10% of the parent compound and 60% of the metabolites which can be found excreted in the urine. The Ex vivo studies had shown that about 93% of the circulating amlodipine drug is bound to the plasma proteins in hypertensive patients. Therefore, the pharmacologic profile of amlodipine is nearly complete absorption, late-peak plasma concentrations, high bioavailability and slow hepatic breakdown. The bioavailability of amlodipine is 60 to 65% and the plasma concentration rise gradually to peak 6 to 8 hours after the administration. There is no significant first-pass metabolism and amlodipine is extensively metabolized in the liver. The volume of distribution of amlodipine is large (21L/kg) and high degree of plasma-protein binding (98%) [5,6].
Impurities of Amlodipine
Amlodipine impurities are listed in Table 1.
Amlodipine is one of the common medications used in Malaysia which can be used to treat the high blood pressure disease. Hypertension is categorized into the top 10 of the common diseases in Malaysia and the patients with hypertensive is keep increasing in these few years. Thus, there are several brands of amlodipine available in Malaysia, hence the purity test of Amlodipine medications available in the market of Malaysia was carried out according to the USP and BP limits. Furthermore, the purity test of the Amlodipine will be determined by the Ultraviolet (UV) Spectrophotometer in the maximum wavelength. Besides, the Fourier-Transform Infrared Red Spectroscopy was carried out in order to determine the presence of functional group in the Amlodipine. Both methods are used to determine the purity of Amlodipine because both instruments are available in the laboratory of Faculty of Pharmacy. There are several brands of Amlodipine tablets available in the market of Malaysia. The concentration determination of each brand of Amlodipine tablets will be compared with the official monographs such as British Pharmacopoeia (BP) and United States Pharmacopoeia (USP). Based on both Pharmacopoeias, the anhydrous or hydrated Amlodipine must contain not less than 97.0 percent and not more than 102.0 percent C20H25CIN2O5 [7,8].
Objectives of the Study
a) To perform the quantitative determination of different brands of amlodipine tablets available in Malaysia by using UV spectrophotometric and Fourier transform infrared red spectroscopy method.
b) To compare the quality of amlodipine tablets available in the market of Malaysia within the brands based on the U.S.P. and B.P. limits.
c) To perform the disintegration test of different brands of amlodipine tablets available in Malaysia.
There were 8 brands of Amlodipine purchased from the different pharmacy outlets in Sungai Petani, Kedah. Each brand of Amlodipine drug was labelled as brand A, B, C, D, E, F, G and H in this research. The further descriptions of the drugs of each brand were recorded in Table 2. Each brand of 20 Amlodipine tablet were weighed and the average weight of tablet was calculated and noted in the research. The diameter and thickness of each Amlodipine tablets was measured and recorded by using digital caliper. All the 20 Amlodipine tablets were crushed into powder using mortar and pestle and kept in a reseal able zipper bag with the labelling. The powdered drug was then kept in a cool and dry place Table 2 [9,10].
Uniformity of Weight of Amlodipine Tablets
a) 20 Amlodipine tablets of each brand were weighed. The average weight was determined, measured and recorded. Each amlodipine tablet was weighed individually and recorded.
b) The percentage of deviation of its weight from the average weight was determined for each tablet by using the formula 1. The criteria for the average weight determination should be specified according to British Pharmacopoeia (B.P.) as mentioned in Table 3.
Formula 1. For uniformity of weight.
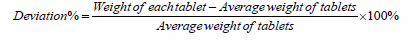
Determination of Diameter and Thickness of Amlodipine Tablets
20 Amlodipine tablets of each brand were measured. The average diameter and thickness were determined, measured and recorded.
Each amlodipine tablet was measured individually and recorded with
digital vernier caliper.
Methyl alcohol / Methanol (EMSURE, Germany), Distilled water freshly prepared in the laboratory, Amlodipine powder, Digital analytical weighing balance. The examples of light source such as Tungsten filament lamps, Hydrogen Deuterium lamps and Xenon flash lamps are suitable used in UV Spectrophotometer. These light sources can cover the whole UV region. The wavelength of Deuterium lamp is from 190 to 350nm and a quartz halogen or tungsten lamp for the visible region is from 350 to 900nm. The UV-Spectrophotometer (Shimadzu 1800 UV/ Visible spectrophotometer). The wavelength of Deuterium lamp is from 190 to 350nm and a quartz halogen or tungsten lamp for the visible region is from 350 to 900nm. The monochromator only allows the radiation with specific wavelength to pass through and leave the exit slit by moving the dispersing element. The role of matt black inside the compartment is helping to absorb the stray light that may enter the compartment. The standard cuvettes have a 10mm or 1 cm path length and are made from quartz in order to ensure good transmittance of UV wavelengths. The photomultiplier is commonly used as a detector in UV-visible spectrophotometer. The photomultiplier consists of a photo emission cathode, several dynodes and an anode [11,12].
a. 20 tablets of Amlodipine were weighed and poured into a 250ml volumetric flask.
b. 50ml of methanol was added into the volumetric flask.
c. The mixture was sonicated for 15 minutes to ensure maximum dissolving of amlodipine tablets with methanol.
d. After sonicating, the volume of the mixture was made up with methanol and it was known as SOLUTION A (stock solution) and filtered.
e. 10ml of solution A was pipetted out into 50ml volumetric flask and made-up volume with methanol solution. This was known as SOLUTION B.
f. 10ml of solution B was pipetted into 50ml volumetric flask and made-up volume with methanol solution. This was known as SOLUTION C.
g. The methanol solution was used as blank solution.
h. The UV-Spectrophotometer was switched on and allowed to stabilize for 20 minutes. ix. Baseline correction was done by using the blank solution and the absorbance of the resulting Solution C was measured at 355nm.
i. The equivalent weight and percentage purity were calculated by using given formula 2 and 3.
Formula 2: Formula for Equivalent Weight.
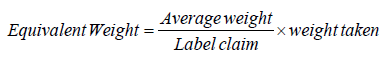
Formula 3: Formula for Percentage Purity of Sample.
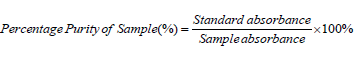
Formula 4: Formula for Percentage Purity of Standard.
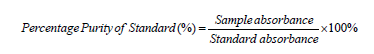
Assay Of Amlodipine Tablets by Using Fourier- Transform Infrared Red Spectroscopic Method (FTIR)
FTIR Spectrophotometer (Perkin Elmer Spectrum Two TM) Ethanol and Amlodipine crushed powder.
The equipment was switched on 20 minutes before functioning in order to stabilize it. The crystal plate was wiped with the ethanol solution. Avoided scratches on the diamond crystal while cleaning the crystal plate. The amlodipine powdered was positioned on the crystal plate and clamped down with pressure arm. The pressure applied was adjusted to the crystal plate using pressure arm to ensure the consistent contact was achieved between the crystal plate and the sample. The sample was scanned using spectrum software. The spectrum was obtained after s canning. The data was analyzed and result was reported. Assay of Amlodipine tablets by using disintegration test Distilled water, Disintegration tester and Thermometer. The disintegration test apparatus consisted of a rack holding 6 glass or plastic tube. Each of the tubes had 10-mesh screen at its bottom. The tubes were raised and lowered at a fixed rate in a water bath maintained at 37±˚C. The six tablets were placed one in each tube along with a plastic disk over each tube. The tubes were allowed to move up and down and the time required for each tablet to disintegrate and pass the screen was recorded [13,14].
The plastic disk did not allow the tablet to float and imparted a slight pressure on the tablets to force any soft mass through the screen. The disintegration tester was set up by filling the distilled water in the two large beaker and there were six small tubes in each big beaker. There was total six tablets of each brand were placed inside the six small tubes and another brand was placed in another six small tubes. Two brands of amlodipine drugs were performed at the same time. The temperature of the distilled water must be maintained at 37˚C (± 1˚C). This was because 37˚C was the normal body temperature of the human beings. The paddle basket-assembly must also have 1cm from the bottom part in order to avoid it touched the big beaker. As a result, all the eight brands of amlodipine tablets used in the research were uncoated tablets and were dissolved within 15 minutes (Table 4) [15,16].
Average weight of different brands of Amlodipine is given in Table 5, and average weight uniformity of different brands of amlodipine in shown in Table 6. Diameter of Amlodipine tablets is given in Table 7. Table 8 shows the average thickness of Amlodipine tablets. Average weight of different brands of amlodipine is shown in Table 5. This table had shown that brand C has the highest weight (202.70mg) per tablet while brand A has the lowest weight (101.60mg) per tablet. The average weight of the tablets for brand B, D, E, F, G and H were 201.20mg, 181.40mg, 166.20mg, 202.60mg, 161.90mg and 104.36 mg respectively. Table 7 has recorded the diameter of Amlodipine tablets in different brands. The average diameter of brand A, B, C, D, E, F, G are 8.60mm, 8.05mm, 11.13mm, 8.07mm, 7.21mm, 8.62mm, 8.77mm, and 8.54mm. The largest average diameter of the brand of Amlodipine tablets are brand C (11.13mm) whereas the brand E (7.21mm) has the smallest average diameter of the tablets. Table 8 had recorded the thickness of amlodipine tablets of different brands. The average thickness of brand A, B, C, D, E, F, G are 2.80mm, 3.30mm, 3.40mm, 2.70mm, 2.57mm, 2.81mm, 2.74mm and 3.06mm. Average thicknesses of the brands of Amlodipine tablets are brand C (3.40mm) whereas the brand E (2.57mm) has the smallest average thickness of the tablets. Table 9 had shown the absorbance of different brand of amlodipine tablets. The U.V absorption of different samples (A-H) is shown in Graphs 1-8. Graph 1 had recorded the UV-absorbance of sample A. The first, second and third reading of standard amlodipine were 0.080nm, 0.081nm and 0.080nm respectively and the average reading of standard was 0.080nm. Total three trials were conducted for the assay of sample A and each trial had three readings. The first, second and third readings in first trial was 0.064nm, 0.067nm and 0.068nm respectively. Average of the reading of first trial of sample A was 0.066nm. The first, second and third readings in second trial was 0.055nm, 0.067nm and 0.069nm respectively. The average of the reading of second trial of sample A was 0.064nm. The first, second and third readings in third trial was 0.073nm, 0.076nm and 0.070nm respectively. The average of the reading of third trial of sample A was 0.073nm [17,18].
UV-Absorbance of Sample B
(Graph 2) The Graph 2 had recorded the UV-absorbance of sample B. The first, second and third reading of
standard amlodipine 0.080nm, 0.081nm and 0.080nm respectively and the average reading of standard was 0.080nm. Total three trials were conducted for the assay of sample A and each trial had three readings. The first, second and third readings in first trial were 0.074nm and the average of the reading of first trial of sample B was 0.074nm. The first, second and third readings in second trial was 0.068nm, 0.070nm and 0.072nm respectively. The average of the reading of second trial of sample B was 0.070nm. The first, second and third readings in third trial was 0.067nm, 0.067nm and 0.071nm respectively. The average of the reading of third trial of sample B was 0.068nm [19,20].
UV-Absorbance of Sample C
(Graph 3) The Graph 3 had recorded the UV-absorbance of sample C. The first, second and third reading of standard amlodipine were 0.080nm, 0.081nm and 0.080nm respectively and the average reading of standard was 0.080nm. Total three trials were conducted for the assay of sample C and each trial had three readings. The first, second and third readings in first trial were 0.066nm, 0.069nm and 0.069nm respectively and the average of the reading of first trial of sample C was 0.068nm. The first, second and third readings in second trial was 0.068nm, 0.069nm and 0.068nm respectively. The average of the reading of second trial of sample C was 0.068nm. The first, second and third readings in third trial was 0.075nm, 0.075nm and 0.070nm respectively. The average of the reading of third trial of sample C was 0.073nm [21,22].
(Graph 4) The Graph 4 had recorded the UV-absorbance of sample D. The first, second and third reading of standard amlodipine were 0.080nm, 0.081nm and 0.080nm respectively and the average reading of standard was 0.080nm. Total three trials were conducted for the assay of sample D and each trial had three readings. The first, second and third readings in first trial were 0.080nm, 0.083nm and 0.083nm respectively and the average of the reading of first trial of sample D was 0.082nm. The first, second and third readings in second trial was 0.084nm, 0.082nm and 0.081nm respectively. The average of the reading of second trial of sample D was 0.082nm. The first, second and third readings in third trial was 0.082nm, 0.083nm and 0.085nm respectively. The average of the reading of third trial of sample D was 0.083nm [23,24].
UV-Absorbance of Sample E
(Graph 5) The Graph 5 had recorded the UV-absorbance of sample E. The first, second and third reading of standard amlodipine were 0.080nm, 0.081nm and 0.080nm respectively and the average reading of standard was 0.080nm. Total three trials were conducted for the assay of sample E and each trial had three readings. The first, second and third readings in first trial were 0.087nm, 0.088nm and 0.083nm respectively and the average of the reading of first trial of sample E was 0.086nm. The first, second and third readings in second trial was 0.079nm, 0.079nm and 0.074nm respectively. The average of the reading of second trial of sample E was 0.077nm. The first, second and third readings in third trial was 0.081nm, 0.082nm and 0.075nm respectively. The average of the reading of third trial of sample E was 0.079nm [25,26].
UV-Absorbance of Sample F
(Graph 6) The Graph 6 had recorded the UV-absorbance of sample F. The first, second and third reading of standard amlodipine were 0.080nm, 0.081nm and 0.080nm respectively and the average reading of standard was 0.080nm. Total three trials were conducted for the assay of sample F and each trial had three readings. The first, second and third readings in first trial were 0.074nm, 0.075nm and 0.073nm respectively and the average of the reading of first trial of sample F was 0.074nm. The first, second and third readings in second trial was 0.074nm, 0.076nm and 0.079nm respectively. The average of the reading of second trial of sample F was 0.076nm. The first, second and third readings in third trial was 0.075nm, 0.075nm and 0.083nm respectively. The average of the reading of third trial of sample F was 0.078nm [27,28].
(Graph 7) The Graph 7 had recorded the UV-absorbance of sample G. The first, second and third reading of standard amlodipine were 0.080nm, 0.081nm and 0.080nm respectively and the average reading of standard was 0.080nm. There were total three trials conducted for the assay of sample G and each trial had three readings. The first, second and third readings in first trial were 0.076nm, 0.068nm and 0.069nm respectively and the average of the reading of first trial of sample G was 0.071nm. The first, second and third readings in second trial was 0.069nm, 0.073nm and 0.069nm respectively. The average of the reading of second trial of sample G was 0.070nm. The first, second and third readings in third trial was 0.079nm, 0.078nm and 0.079nm respectively. The average of the reading of third trial of sample G was 0.079nm [29,30].
UV-Absorbance of Sample H
(Graph 8) The Graph 8 had recorded the UV-absorbance of sample H. The first, second and third reading of standard amlodipine were 0.080nm, 0.081nm and 0.080nm respectively and the average reading of standard was 0.080nm. There were total three trials conducted for the assay of sample H and each trial had three readings. The first, second and third readings in first trial were 0.077nm, 0.074nm and 0.079nm respectively and the average of the reading of first trial of sample H was 0.077nm. The first, second and third readings in second trial was 0.080nm, 0.081nm and 0.080nm respectively. The average of the reading of second trial of sample H was 0.080nm. The first, second and third readings in third trial was 0.073nm, 0.073nm and 0.070nm respectively. The average of the reading of third trial of sample H was 0.072nm [31,32].
The percentage of purity determination of different brands of amlodipine by using UV Spectrophotometric method is tabulated in Table 10. The percent purity of standard amlodipine is 95%. There were total three trials conducted for each assay of each brand of the sample. The percentage of purity of first, second and third trials of sample A were 121.21%, 125.00% and 109.59%. Average percentage of purity of sample A was 118.60%. The percentage of purity of first, second and third trials of sample B were 108.11%, 114.29% and 117.65%. Average of percentage of purity of sample B was 113.35%. The percentage of purity of first, second and third trials of sample C were 117.65%, 117.65% and 109.59% respectively and the average percentage purity of sample C was 114.96%. Furthermore, the percentage of purity of sample D for three trials of first and second trials were 97.56% and third trial was 96.39% The average percentage of purity of sample D was 97.17%. The percentage of purity of first, second and third trials of sample E were 93.02%, 103.90%, 101.27%. Average percentage of purity of sample E was 99.40%. The percentage purity of first, second and third trials of sample F were 108.11%, 105.26% and 102.56% respectively. The average of the percentage of purity of sample F was 105.31%. The first, second and third trials of the percentage of purity of sample G were 114.29%, 101.27% and 109.41%. Average percentage of purity of sample G was 109.41%. The percentage purity of first, second and third trials of sample H were 103.90%, 100% and 111.11%. The average percentage of purity of sample H was 105.00% [33].
Table 10: Percentage of purity determination of different brands of Amlodipine tablets by using UV Spectrophotometric method.
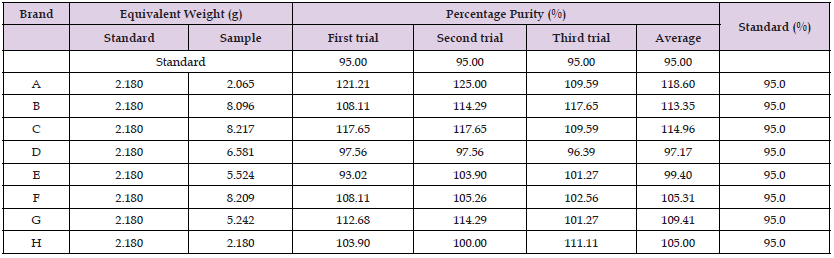
(Graph 9) The comparison of percent purity between standard and sample of Amlodipine by using UV Spectrophotometric method were recorded in graph 9. The percent purity of standards for all the samples was 95%. Moreover, the average percent purity of sample A, B, C, D, E, F, G and H were 118.60%, 113.35%, 114.96%, 97.17%, 99.40%, 105.31%,109.41% and 105.00% respectively. Based on the Graph 4, sample D had the most purity with standard amlodipine while sample A had the least purity with standard amlodipine. According to the British Pharmacopoeia (BP), the percent purity of amlodipine in tablet should be within the range of 95.0% to 105.0% and as the result had shown that only sample D and E had passed the test. Besides, the United State Pharmacopoeia (USP) had set the limit of amlodipine in tablet should contain not less than 90.0% and not more than 110% of the labelled amount of amlodipine and the sample D, E, F, G and H had passed the test (Table 11) [34,35].
Table 11: Results of different brands of Amlodipine tablets using Fourier-Transform Infrared Red Spectroscopic method.
Comparison of the IR Spectrum of Different Brands of Amlodipine Tablets
(Graph 10) showed the comparison of the IR spectrum of different brands of amlodipine tablets. The alkyne group was present in the IR spectrum of brand H in the wavelength of 3261.29 cm-1. Moreover, there was the presence of alkane group in the wavelength of 2899.39 cm-1. The peaks that found between 2500 cm-1 to 2000 cm-1 and 2000 cm-1 to 1600 cm-1 were indicated as triple bond and double bond region. The alkene group such as C=C unconjugated was found in the wavelength of 1672.52 cm-1. Lastly, the organic halide was found in the wavelengths of 630.93 cm-1, 603.76 cm-1, 551.23 cm-1 and 468.02 cm-1. Based on the comparison, the spectra were like each other brands except brand H. Brand H was the combination of drugs between amlodipine and perindopril and hence it showed more peaks and bonding of stretching between 1500 cm-1 to 1000 cm-1 when compared with other seven brands of amlodipine tablets [36,37].
Table 12 showed the time taken for the amlodipine tablets to disintegrate using disintegrate tester. All the different brands of Amlodipine tablets were disintegrated within 15 minutes. This shown that the drugs were uncoated tablets and hence it can be absorbed rapidly through oral route. The temperature of the distilled water was maintained at 37˚C (±1˚C) [38].
The objective of this research was to evaluate the percent purity of different brands of amlodipine tablets available in local market of Malaysia by using UV-Spectrophotometric method and FTIR Spectroscopy methods. All the limitations that carried out during the research were according to the B.P and U.S.P Pharmacopoeia. There was total 8 brands of amlodipine tablets available in the pharmacy outlets from the area of Sungai Petani, Malaysia. The powdered drugs were kept in the sealed bags respectively with the labelling to avoid the powder contact with the moisture which may lead the instability of the drug sample and inaccurate result. The powdered drugs with sealed bags were kept in a refrigerator in order to increase the shelf life of the drug samples. The average weight of different brands of amlodipine drug was recorded. Result shows the brand C had the highest weight and brand A had the lowest weight. Therefore, brand C may be contained the most excipients while brand A contained the least excipients in the formulation. The uniformity of weight of different brands of amlodipine tablets was recorded. Based on the limit of deviation of individual weight from the average weight of the amlodipine tablet, the amlodipine tablets of different brands were under the category of ‘80mg to 250mg’ and all the brands of amlodipine tablets passed the weight uniformity test.
Weight variation test plays a vital role in the pharmaceutical industry to followed the Good Manufacturing Practice (GMP) and all the tablet contains the same amount of drug substance with a defined allowed variation with in a batch. Moreover, the average diameter of different brands of Amlodipine tablets were checked. Brand C had the largest diameter whereas brand E had the smallest diameter compared to the 8 brands of amlodipine tablets. Brand C had the largest thickness whereas brand E had the smallest thickness within the 8 brands of amlodipine tablets. Both the diameter and thickness of amlodipine tablets were measured by using digital caliper. The result of percent purity between standard and sample by using UV- Spectrophotometric method was recorded and the comparison of percent purity between standard and sample of amlodipine by using UV-Spectrophotometric method was also recorded. According to the United State Pharmacopoeia (USP), the percent purity of amlodipine tablets should contain not less than 90.0 % and not more than 110.0% of the labelled amount of amlodipine and only the sample D, E, F, G and H were passed the test. Besides, according to the British Pharmacopoeia (BP), the percent purity of Amlodipine tablets should be within the range of 95.0 % to 105.0 % and only sample D and E passed the test.
This resulted that brand D was within the limit specified by U.S.P and B.P and the best among the eight brands of amlodipine used in this research. UV-Visible Spectroscopy is based on the absorption of ultraviolet light or visible light by the chemical compounds, resulting in the production of distinct spectra. The principle of UV-Vis Spectrometer was studied under Beer Lambert law. The Beer- Lambert’s law is formulated below:

Io = intensity of incident light
IT = intensity of transmitted light
K = extinction coefficient
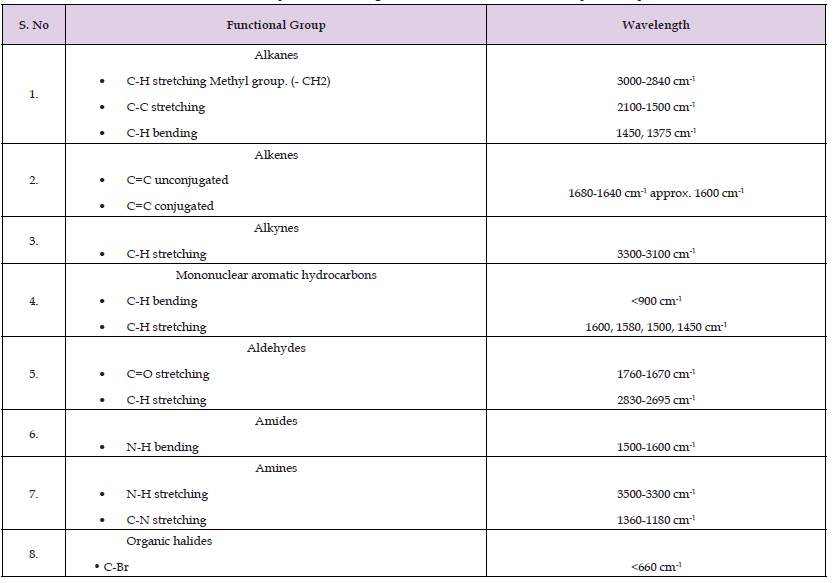
Based on the IR spectrum of each brand of amlodipine tablets, there were several functional groups present in the chemical structure of Amlodipine. For example, alkynes of C-H stretching methyl group, alkanes of C-H stretching, triple bond and double bond region, alkene group of C=C unconjugated and organic halides of C-Cl were present in the IR spectrum of Amlodipine drugs. The comparison of the IR spectrum of different brands of Amlodipine tablets was carried out. All the spectrum of eight brands of Amlodipine tablets was almost similar to each other brands except brand H. There were some peaks of stretching found between 1500 cm-1 to 1000 cm-1 compared to other brands of Amlodipine drugs. This was because brand H was the combination of drugs within amlodipine and perindopril and hence there were other functional groups present in the sample. The disintegration tester was set up by filling the distilled water in the two large beakers and there were six small tubes in each big beaker. There was total six tablets of each brand were placed inside the six small tubes and another brand was placed in another six small tubes. Two brands of amlodipine drugs were performed at the same time. The temperature of the distilled water must be maintained at 37˚C (± 1˚C). This was because 37˚C was the normal body temperature of the human beings. The paddle basket-assembly must also have 1cm from the bottom part in order to avoid it touched the big beaker. As a result, all the eight brands of amlodipine tablets used in the research were uncoated tablets and were dissolved within 15 minutes [39].
In nutshell, brand D has the best percent purity among the eight brands of amlodipine tablets used in this research. Besides, brand A had the least percent purity among these eight brands of amlodipine used in this research. Based on the U.S.P and B.P. Pharmacopoeia, the brand A was the worst among all the eight brands of amlodipine as it had the limits more than 110%. Next, the UV Spectrophotometer and FTIR-Spectrometer were useful for the assay of amlodipine tablets. The assay of the pharmaceutical products should be always performed to ensure the product was maintained in safety, quality and efficacy condition. Finally, the disintegration test was also helpful in the efficacy of the pharmaceutical product. All the eight brands of amlodipine tablets had passed the quality control of the disintegration test which can be disintegrated in distilled water with the temperature of 37˚C (±1˚C) within 15 minutes and hence it indicated that the orally taken drug can be easily disintegrate into human body.
The authors are much grateful to the Faculty of Pharmacy, AIMST University, Malaysia for funding and providing all facilities to carry out this research project.
Authors declare that there is no conflict of interests.
Thanks to science officers, Ms. Amalina and Ms. Jaya of MDL 4.


