Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Michel Kere1,2, Neng Wen Lo3, Yang Kwang Fan1, Hsing I Chiang1* and Jyh Cherng Ju1,4,5,6*
Received: October 05, 2023; Published: October 16, 2023
*Corresponding author: Hsing I Chiang, Department of Animal Science, National Chung Hsing University, 250 Kuo Kuang Road, Taichung 402, Taiwan
Jyh Cherng Ju, Department of Animal Science, National Chung Hsing University, 250 Kuo Kuang Road, Taichung 402, Graduate Institute of Biomedical Sciences, China Medical University, Taichung, Translational Medicine Center, China Medical University Hospital, Taichung, Department of Bioinformatics and Medical Engineering, Asia University, Taichung 41354 Taiwan
DOI: 10.26717/BJSTR.2023.53.008384
The effects of vascular endothelial growth factor A (VEGFA) supplementation at 5 ng/mL in IVP media on the development of porcine embryos were assessed in this study. Addition of VEGFA in the IVM medium increased monospermic (85.1%) and normal fertilization (68.0%) rates compared to those of the control groups (74.7 and 57.7%, respectively, P < 0.05). Both IVM and IVC media supplemented with VEGFA promoted a greater blastocyst rate compared to the control group (46% vs. 31%, P < 0.05). Also, the total cell number per blastocyst increased in the presence of VEGFA in IVC (74.4 ± 10) or both (81.8 ± 8.2), compared to control during IVM or IVC (62 ± 11, P < 0.05). The apoptotic indices were reduced when VEGFA was contained in both IVM and IVC (2.4 ± 0.1%) media compared to the control group (5.5 ± 1.4%). Moreover, VEGFA increased GSH level by 1.4 to 3.4 folds, where it concomitantly decreased ROS by half fold in matured oocytes, 4-celled embryos and blastocysts. Taken together, the positive effects of VEGFA on oocyte maturation and embryonic development may be carried over from IVM to post-IVF development. It not only functions as a growth/paracrine factor, but also helps in maintaining the ROSGSH homeostasis by acting as an antioxidant during early embryogenesis in porcine species.
Keywords: Embryos; GSH; In Vitro Fertilization; Porcine; ROS; VEGFA
Abbreviations: VEGFA: Vascular Endothelial Growth Factor A; IVP: In Vitro Production; IVM: In Vitro Maturation; COCs: Cumulus-Oocyte Complexes; TdT: Terminal Deoxynucleotidyl Transferase; ANOVA: Analysis of Variance; GLM: General Linear Model
The porcine embryo production in vitro (IVP) has been increasingly attractive as a tool for biochemical research; it also bears great promise as a study model for both embryonic development and stem cell technologies [1,2]. In vitro maturation (IVM) of oocytes from antral follicles is a valuable source to increase the number of fertilizable oocytes alternative to the in vivo oocytes [3]. Intensive efforts have been made to optimize the in vitro production (IVP) system of porcine embryos, but the yield and the quality of IVP embryos are still low compared to their in vivo counterpart [4,5]. Oocyte and embryo quality exerts a significant influence on the pre- and post-implantation development of embryos [6,7]. A great majority of improvements in oocyte development was based on the studies starting from the IVM oocytes to the developing blastocyst stage [3,8,9].
By ameliorating the suboptimal conditions for oocyte maturation and embryo culture systems, the pattern of gene expression could mimic closely that of the in vivo conditions, leading to increased production of quality blastocysts [10,11]. Although strictly defined media for oocyte IVM and embryo IVP in domestic species are largely available, porcine oocyte- and/or blastocyst-promoting agents to improve the yield and quality of IVP embryos are still under investigation [12]. The efforts including addition of growth factors, cytokines, vitamins and/or amino acids to culture media have reached little consensus [4,13,14].
The VEGF signaling are found in animal reproductive system and these molecules function as paracrine/autocrine factors to promote cell proliferation, survival and steroid hormone production [15-17]. In porcine system, previous studies have demonstrated that VEGF signaling molecules, including VEGF and its receptor VEGFR2 are expressed in the ovary and at various stages of parthenogenetic embryos. Moreover, addition of VEGFA to the IVM or IVC medium promoted porcine oocyte maturation and enhanced subsequent parthenogenetic and cloned embryo development [18-20]. There are some fundamental differences between fertilized and parthenogenetic embryos. In fact, IVF embryos add enhanced blastocyst development and diploid chromosomes, the parthenotes have reduced TE cells counts and a higher relative proportion of apoptotic cells in inner cell mass and TE [21-24] compared to the fertilized embryos. Also parthenotes do not develop to term unless they have been genetically manipulated to express specific imprinted gene [25,26]. In the present study, we further investigated the joint effects of VEGFA treatments in both IVM and IVC media on the development of porcine IVF embryos with a similar protocol as in previous study.
Chemicals
All chemicals used in this study were purchased from Sigma Chemicals (St. Louis, MO, USA), unless otherwise specified. Recombinant Human VEGFA165 protein, 293-VE (R&D System, Minneapolis, MN) was used in the IVM and IVC media.
Oocyte Collection, Maturation and Embryo Culture
Swine ovaries sampled from a local abattoir nearby Taichung city (Taichung Meat Market, Co. LTD.). Procedures for collection of ovaries and aspiration of follicles were described previously [27]. In brief, freshly harvested cumulus-oocyte complexes (COCs) were rinsed several times and cultured for 22 h in the NCSU-23 medium supplemented with 10% porcine follicular fluid, FSH (10 IU/mL) and hCG (10 IU/mL). The COCs were further cultured for an additional 22 h in the same medium without hormones.
Measurement of ROS and Intracellular GSH Levels
The matured oocytes, 4-celled embryos and blastocysts were sampled to determine intracellular ROS and GSH levels by the protocol described previously [27]. Briefly, H2DCFDA (2’, 7’-dichlorodihydrofluorescein diacetate; Invitrogen, Eugene, Oregon, USA) and CellTracker Blue CMF2HC (4-chloromethyl-6.8-difluoro-7-hydroxycoumarin; Invitrogen, Eugene, Oregon, USA) were used to detect intracellular ROS by green fluorescence and GSH level by blue fluorescence, respectively. Ten oocytes from each treatment group were incubated for 30 min in NCSU-23 supplemented with 10 μM H2DCFDA and 10 μM CellTracker. After incubation, oocytes were washed with DPBS containing 0.1% (w/v) PVA and placed into 10 µl droplets, and the fluorescence was observed under an epifluorescence microscope (TE300; Nikon, Tokyo, Japan) with UV filters (460 nm for ROS and 370 nm for GSH). Fluorescent images were saved as graphic files, and their fluorescence intensities were analyzed with ImageJ software (Version 1.44; National Institutes of Health, Bethesda, MD, USA). All image data were standardized by the untreated control oocytes for statistical analysis.
Sperm Preparation and in vitro Fertilization
Fertilization process has been described by Nguyen, et al. [10]. Briefly, diluted semen from two boars of proven fertility was supplied by a porcine artificial insemination center (Taichung, Taiwan), stored at 15°C and used for 2 days. Motile sperm were obtained by centrifugation at 700×g with Percoll discontinuous gradient (2 mL 45% over 2 mL 90%ofPercoll) for 15 min. Spermatozoa were collected from the bottom of the 90% fraction and washed in DPBS supplemented with 10% FBS, followed by centrifugation at 100×g for 5 min. After centrifugation, the sperm pellet was resuspended in modified Trisbuffered medium (mTBM) containing 113.1 mM NaCl, 3 mM KCl, 7.5 mM CaCl2.2H2O, 20 mM Tris, 11 mM glucose, 5 mM sodium pyruvate, and 0.2% (w/v) BSA. After 44 h of maturation, oocytes were freed from cumulus cells and matured oocytes were washed three times in mTBM that had been pre-equilibrated for 12 h at 39°C under a 5% CO2incubator. After washing, groups of 30 oocytes each were randomly placed into 45 μL droplets of mTBM medium covered with pre-warmed mineral oil. After dilution (10X), 5 μL of sperm suspension was added to a 45 μL drop of fertilization medium (mTBM) to give a final sperm concentration of 5×105 sperm/mL. The gametes were co-cultured for 6 h at 39°C in an incubator containing 5% CO2 in humidified air.
Evaluation of Fertilization Rates
An experiment was designed to evaluate the fertilization rate. At 10 h post-insemination, parts of oocytes were fixed in DPBS containing4% paraformaldehyde. The fixed oocytes were subjected to triple staining and observed under an epifluorescence microscope (TE300; Nikon, Tokyo, Japan) for nuclear evaluation, and were classified into three categories, i.e., unfertilized oocytes, monospermic oocytes (two pronuclei, 2PN), and polyspermic oocytes (with 2PN or more plus swollen sperm head); the number of sperm that had penetrated oocytes was also evaluated. The experiment was done according to the process described by Nguyen, et al. [28].
Embryo Culture
After 6 h, oocytes were removed from the fertilization medium, and washed three times in porcine zygote medium-3 (PZM-3) to remove excessive sperms. The presumptive zygotes were then cultured in 100 μL droplets (20 to 30 oocytes per droplet) of PZM-3 covered with mineral oil for 7 days. Cleavage and blastocyst rates were evaluated under a stereomicroscope at day-2 and day-7 after insemination (insemination day = day-1).
Apoptotic Cell Measurement and Apoptosis Indices
The blastocysts were subjected to Terminal deoxynucleotidyl transferase (TdT) d’UTP Nick-End Labeling (TUNEL) staining and cell counting for total cell number and apoptotic cells as previously described [10,27]. The apoptotic index was calculated as follows: apoptotic index (%) = (number of positive nuclei/total cell number) × 100.
Statistical Analysis
All data were subjected to analysis of variance (ANOVA) using the General Linear Model (GLM) procedure in SAS version 9 (SAS Institute, Cary NC, USA), followed by Tukey’s test. Percentile data were arcsine transformed before statistical analysis and the probability at P < 0.05 was considered significantly different between treatment groups.
Sperm Penetration Rate
An experiment was designed to evaluate the normal fertilization rate of pig oocytes after IVM culture. The results are presented in (Table 1) using 141 oocytes for control groups and 182 oocytes for 5 ng/mL VEGFA supplemented groups. The sperm penetration rates were not different among treatment groups (78 vs. 80%, P < 0.05). In the presence of VEGFA in the IVM medium, increased monospermic rates was observed as compared to those of the control group (85% vs. 75%, P < 0.05). Similarly, a significant improvement of normal fertilization (Figure 1) rate was also found in the presence of VEGFA during IVM compared to the non-VEGF treated control group (68% vs. 58%, P < 0.05).
Table 1: Effects of VEGFA supplementation in the maturation medium on in vitro fertilization of porcine oocytes.
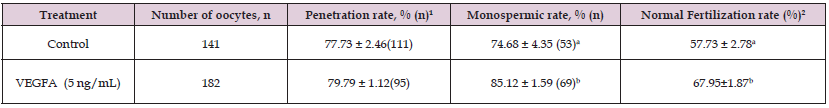
Note: Numbers in the parentheses represent the number of oocytes examined. Data are mean ± SEM from four replicates. a,bWithin a column, means without the same superscript differ (P < 0.05). 1Monospermic rate (%): number of monospermic oocytes/total penetrated oocytes × 100. 2Normal fertilization rate (%): number of monospermic oocytes/total inseminated oocytes × 100. IVM: in vitro maturation; IVC: in vitro culture.
Cumulative Effects of VEGFA on Embryos’ Development During IVM and IVC
We tested the IVM and IVC media in the presence or absence of VEGFA with the end-point development of porcine embryo in vitro. As shown in (Table 2), presence of VEGFA in both IVM and IVC media enhanced blastocyst formation rate (46%) compared to the control group (31%, P < 0.05). The total cell number per blastocyst was increased when VEGFA was supplemented in both IVM and IVC media (81.8 ± 8.2) or in IVM medium alone (74.41 ± 10), in contrast to that from the control group without VEGFA during IVM and IVC (62 ± 11, P < 0.05). The apoptotic indices were reduced in all VEGFA-treated groups compared to those in the control group (P < 0.05).
Table 2: Effects of VEGFA supplementation in the maturation and the culture media on the development of porcine embryos in vitro.

Note: Data are mean ± SEM from three replicates. Numbers in the parentheses represent the number of embryos in each category. a,b Within a column, means without the same superscript differ (P < 0.05). +: Medium supplemented with VEGFA. -: Medium without VEGFA supplementation. *TCN: total cell number.
Effects of VEGFA on GSH and ROS Levels of Matured Oocytes
To verify how VEGFA relieves oxidative stress from oocytes, we checked its optimized concentration effect on the ROS/GSH balance. The micrograph represents the intracellular ROS/GSH of the matured oocytes, 4-cell stage embryos and blastocysts (Figure 2A). Results showed that significant increases of GSH by 3.4, 1.5 and 1.4 folds, respectively, when 5 ng/mL of VEGFA was supplemented in matured oocytes, 4-celled embryos and blastocysts compared to the control group. Concomitantly, ROS levels were reduced by 0.5, 0.45 and 0.6 folds, respectively, in matured oocytes, 4-cell stage embryos and blastocysts in the above culture conduction (Figure 2B). The overall trend was an increase (P < 0.05) of intracellular GSH levels and decreased (P < 0.05) ROS levels when media were supplemented with 5 ng/mL of VEGFA.
In the present study, starting with similar penetration rates among all treatment groups, the IVM medium containing VEGFA had greater monospermic rate (and normal fertilization) compared with the control group. The highest blastocyst development, total cell numbers per blastocyst and reduced apoptotic indices were observed when VEGFA was supplemented in both IVM and IVC media. All VEGFA–treated embryos had reduced apoptotic indices. In addition, VEGFA significantly relieved oocytes from oxidative stress by maintaining the ROS/GSH homeostasis.
The limited knowledge on the regulation of oogenesis, folliculogenesis and the required conditions for oocytes to undergo proper growth, differentiation and maturation, is one of the major factors causing the failure in obtaining viable offspring from in vitro matured oocytes in both domestic species and humans [3]. Higher levels of apoptosis were observed in IVP equine, porcine and bovine blastocysts compared to their in vivo counterparts [29]. The total number of cells in each blastocyst produced in vitro ranges from 58 to 139 which are much less than 150 to 250 cells in the blastocyst obtained in vivo [21]. In the present study, an average of 81 cells per blastocyst was obtained with porcine IVF embryos and constitute a good progress regarding porcine embryos IVP. In fact, porcine IVF embryos rarely reached 77 cells per embryos [2,5,18,21,28]. Unlike other mammalian species, the IVM and IVC platforms in porcine system remain to be further improved [5,30,31].
The improvements were observed on the quality of cloned embryos and parthenotes in pigs when media are supplemented with VEGFA whose effects can be extended or carried over to the IVF embryos. Our previous study reported signaling of VEGFA and its receptor 2 along with follicle growth up to the preimplantation embryo development [19]. Moreover, the addition of VEGFA in culture media improved the extrusion of polar body and parthenote quality [19]. Such paracrine factor improved IVM of porcine cumulus-oocytes complexes maturation [18,19] and the subsequent development of cloned embryos [19,32]. Thus, it is evident that VEGFA functioning as promoter of cell survival and proliferative factor is sustained [33].
Although several exogenous factors are used in IVP media, culture conditions induce excessive production of ROS by embryos. External protection occurs in follicular and tubal fluids, which mainly consists of non-enzymatic antioxidant, such as hypotaurine, taurine and ascorbic acid. Internal protection mainly comprises antioxidant enzymes including superoxide dismutase, glutathione peroxidase and γ-glutamyl cysteine synthetase. The GSH content of oocytes is associated with the male pronuclear formation and blastocyst development, as well as the total cell number of each blastocyst [27,34,35]. An increase in GSH storage is observed when antioxidants, such as ascorbic acid [27,34,36] and cysteamine [36], are supplemented in the oocyte maturation medium.
In this study, the improved fertilization rate was conceivably attributed to an enhanced cytoplasmic maturation of oocyte conferred partly by the increased storage of GSH and the reduced ROS contents of porcine oocytes. Polyspermy, referring to fertilization involving more than one sperm [37], is a pathological phenomenon that leads to developmental failure in various mammalian species. Within the female reproductive tract, several mechanisms exist in regulating the number and quality of spermatozoa in order to minimize the occurrence of polyspermy [38]. Also, an improved embryo culture environment allowed an increase of normal fertility, total cell number with reduced apoptotic indices denoting VEGFA’s involvement in cytoprotective and proliferative effects on early embryogenesis. Our previous study also showed that PI3K/AKT and ERK/MEK mediated the effect of VEGF in vitro, as well as that reduced Caspase-3 activation was observed [19]. Those cytoprotective and proliferative effects of VEGFA were reported [39-42] and the PI3K/AKT and ERK/MEK signaling pathways were found to be involve in these processes [43-46]. In tumor cells, VEGF activates the prosurvival signaling in response to increasing ROS content in the culture environment [45]. In addition to the proliferative and cytoprotective effects, the present study is the first to demonstrate VEGFA supplementation positively affected the GSH and ROS homeostasis in oocytes and developing embryos [47].
Exogenous human recombinant VEGFA supplementation at a concentration of 5 ng/mL in IVP media improved normal fertilization rate, followed by subsequent improved quality of developing embryos. Apart from acting as a paracrine/autocrine factor, VEGFA showed a strong antioxidant capability in the IVP system in protecting gametes and embryos. VEGFA can therefore be added to IVP system for production of competent embryos for agriculture production improvement or biomedical investigation such as stem cells, transgenes, xenograft studies. Further investigations are required to understand the molecular mechanisms behind the action of VEGFA related to its signaling pathways.
Conceptualization, J.-C.J. and Y.-K.F.; investigation, M.K.; Conceptualization, J.-C.J. and Y.-K.F.; formal analysis, H.-I.C. and M.K.; investigation, M.K.; resources, N.-W.L.; writing—original draft preparation, M.K.; writing—review and editing, J.-C.J., N.-W.L., H.-I.C. and Y.-K.F.; project administration, J.-C.J. All authors have read and agreed to the published version of the manuscript.
This research was funded grants from China Medical University and Hospital (CMU108-MF-46; CMU109-MF-100; DMR-109-125) and the Ministry of Science and Technology (MOST 106-2313-B-039-003-MY2 and MOST108-2313-B-039-002), Executive Yuan, Taiwan, Republic of China.
Not applicable.
Not applicable.
We wish to thank the Department of Animal Science, National Chung Hsing University for kindly providing all the facilities for and assistance with the experimentation. We are grateful to Taichung Meat Market, Co. LTD., a local abattoir in Taichung City, continuously supporting us with pig ovaries for the entire study.
The authors declare no conflict of interest.


