Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Natalia Cichowska-Cwalińska1,3, Marta Popęda2, Magdalena Dróżka1, Ewa Pawłowska1, Michał Bieńkowski2 and Renata Zaucha1*
Received: September 19, 2023; Published: October 02, 2023
*Corresponding author: Renata Zaucha, Department of Oncology and Radiotherapy, Medical University of Gdańsk, Gdańsk, Poland
DOI: 10.26717/BJSTR.2023.53.008347
Aim: The definitive radiation (RT) doses for patients with head and neck cancers (HNC) are standardized
and guided primarily by TNM status. We hypothesized that baseline indicators of immune response,
such as neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) correlate with RT
response. Our study aimed to examine the relationship between primary tumor volume (GTV-P) and
NLR/PLR values in nonmetastatic, p16-negative, oral cavity (OC), pharyngeal (PX), and laryngeal (LC)
cancers.
Methods: We performed a retrospective single-center analysis involving HNC patients who underwent
definitive RT. NLR and PLR were calculated based on pretreatment complete blood count (CBC). The
study explored overall survival (OS) relative to the GTV-P, baseline inflammatory markers and other
characteristics.
Results: Study group included 240 patients (OC, n=46; PX, n=75; LC, n=119). The cumulative 5-year
overall survival (5YOS) rate was 44%. In OC, PX, and LC cases, elevated baseline NLR/PLR values were
linked to over a twofold reduction in 5YOS. The median GTV-P, along with NLR/PLR values emerged
as robust prognostic markers. The best long-term outcomes were observed in low-NLR/PLR in small
tumors. Small tumors with high-NLR/PLR values had a similarly unfavorable prognosis as opposed to
larger tumors with low-values. Multivariate analysis identified high-PLR in LC (p=0.01), and high-NLR in
PX as indicators of poor prognosis.
Conclusion: The outcomes of OC, PX, and LC treated with definitive (C)RT are notably influenced by
peripheral immune markers. High-NLR/PLR values should be considered in risk assessment strategies.
For patients with High-NLR/PLR, further research is needed on the benefit of intensifying treatment.
Keywords: NLR; PLR; HNC; Radiotherapy; Tumor Volume
Abbreviations: AJCC: American Joint Committee on Cancer; CBC: Circulated Blood Cells; Cls: Confidence Intervals; (C)RT: (Chemo)Radiotherapy; DDP: Cisplatin; ECOG: Eastern Cooperative Oncology Group Scale; HRs: Hazard Ratios; HNC: Head And Neck Cancer; ICRU: International Commission On Radiation Units & Measurements; IMRT: Intensity Modulated Radiation Therapy; LC: Laryngeal Cancer; NLR: Neutrophil to Lymphocyte Ratio; OC: Oropharyngeal Cancer; OS: Overall Survival; PD-L1: Percentage Of Cells Expressing Ligand For Programmed Death 1; PLR: Platelet To Lymphocyte Ratio; PX: Pharyngeal Cancer; RFR: Regional Failure Rate; ROC: Receiver Operating Characteristic; VMAT: Volumetric Modulated Arc Therapy; mGTV-P: Median Gross Tumor Volume – Primary Site; mOS: Median Overall Survival
Every year, squamous cell head and neck cancer (HNC) is diagnosed in approximately 700 000 new patients, contributing to 350 000 deaths globally [1]. Surgery or definitive radiotherapy (RT), with or without concurrent chemotherapy (CRT), remains the established gold standard for radical treatment [2-4]. Despite employing combined treatment approaches, patient outcomes remain less than satisfactory, with only half surviving beyond five years. The survival of patients who undergo RT for squamous HNC of the oral cavity, oropharynx, hypopharynx and larynx is influenced by a range of patient and cancer-related characteristics. Key risk factors include the primary tumor›s location, size, nodal disease burden, nutritional status, patient performance status (ECOG) and age [5]. These attributes impact treatment decisions. TNM classification, while prognostically predictive based on clinical/radiological tumor and regional lymph node features does not consider the diverse tumor volumes often found at the same T (primary tumor) stage [6,7]. An illustrative example is superficial neoplasm, which often shares the same T classification as more invasive tumors. The understanding of biological and molecular prognostic markers in this disease is limited compared to better researched cancers like lung or breast cancer. In recent years human papillomavirus (HPV) positive PX tumors were shown to exhibit distinct epidemiology and notably better prognosis than HPV-negative counterparts.
This led to modifications in the 8th TNM AJCC classification. The exploration of treatment deintensification is an actively pursued avenue in research. However, this approach remains in the early phases. Nevertheless, these studies have shown encouraging rates of progression-free survival (PFS) despite employing reduced radiation therapy (RT) dosages [8,9]. Another research area within HNC evolves around the inflammatory responses within tumor tissues. Inflammation, a hallmark of cancer, influences cancer development, progression, and response to treatment [10]. The cancer-induced alterations in the immune system precipitate variations in the composition of distinct populations of leukocytes and platelets within the tumor microenvironment. The NLR and PLR are used as surrogate indicators of the extent of inflammation. These ratios have demonstrated prognostic significance across several solid tumors, including lung, head and neck, breast, and colorectal cancers [11-13]. Clinical investigations have delved into the utilization of NLR and PLR as prognostic indicators for HNC. Despite encouraging findings, a consensus on their clinical application remains elusive. In this ongoing study, we sought to evaluate the prognostic implications of baseline inflammatory markers (NLR, PLR) and their correlation with the volume of the irradiated primary tumor (GTV-P). We focused on outcomes in nonmetastatic, p16-negative, oral cavity (OC), pharyngeal (PX), and laryngeal (LC) cancers managed with definitive (C)RT.
Patients, Data Collection, and Ethics Statement
This retrospective study involved 240 consecutive patients diagnosed with nonmetastatic OC (n=46), oro-PX, and hypo-PX (n=75), and LC (n=119) amenable for radical (C) RT and treated with RT at the Department of Clinical Oncology and Radiotherapy of the Medical University of Gdansk between the years 2012 and 2018. The inclusion criteria encompassed:
1. p16-negative squamous cell carcinomas,
2. Treatment with definitive (C)RT,
3. TNM staging - T1-4, N0-3, M0 (reassessed retrospectively
according to TNM 8th ed).,
4. Availability of pretreatment CT scans for RT planning, and
5. Available baseline blood counts (CBC) obtained within 15
days before the commencement of treatment. The patients›
clinical characteristics were derived from their medical
records and meticulously reviewed by two independent
researchers (NC, MD).
The clinical data included: age at diagnosis, primary tumor site (oral cavity, oropharynx, hypopharynx, and larynx), TNM stage, ECOG, comorbidities, cumulative cisplatin dose, treatment complications during (C)RT requiring antibiotic or steroid therapy, scheduled completion of RT; and the 5-year outcome (cancerrelated or other causes of death). The GTV-P volumes were extracted from the pretreatment CT scans for RT planning, utilizing the Eclipse system. Ethical approval for this study was granted by the Bioethical Committee of the Medical University of Gdańsk (NKBBN/357-298/2016) and was carried out in alignment with the Declaration of Helsinki. Due to the retrospective nature of the study, the requirement for informed consent was waived.
Treatment
All patients received treatment in accordance with the departmental guidelines based on international recommendations and multidisciplinary decisions [14-17]. Board-certified specialists in head and neck radiation oncology contoured the required target volumes. Subsequently, they prescribed radiotherapy doses in strict adherence to the guidelines set out in the International Commission on Radiation Units & Measurements (ICRU) report 83. Medical physicists prepared treatment plans using the Eclipse system. Photon radiotherapy was administered using intensity modulated radiation therapy (IMRT) or volumetric modulated arc therapy (VMAT). Simultaneous integrated boost (SIB) was used in all patients with level doses: 66 Gy, 63 Gy, 60 Gy and 54 Gy. The dose prescribed to the GTV-P was 66 Gy given in 5 weeks (2.2 per fraction). For early glottic cancers hypofractionated regimens were used: T1 tumors 63 Gy/28 fx and T2 tumors 65.25 Gy/29 fx. (T1N0, n=21; T2N0, n=28). Concurrently, chemotherapy consisting of cisplatin (DDP) at a dose of 100 mg/m2 i.v. every three weeks or 40 mg/m2 i.v. once a week was administered. Three patients with renal insufficiency were prescribed carboplatin. The assessment of OS duration encompassed the period between commencement of treatment and the date of death or the last follow-up contact for patients still alive at that point.
Statistical Analysis
The data was analyzed and visualized using the R computing environment (4.1.2) [18]. Receiver-operator-characteristic (ROC) analysis was used to dichotomize NLR and PLR values for each specific tumor site (median Area Under Curve (AUC) 0.61, range 0.533-0.656). For the examination of tumor volume, patients were segregated based on the median GTV-P to ensure comparable groups sizes. The correlation with OS was assessed through univariable and multivariable Cox regression models, reporting Hazard Ratios (HRs) along with corresponding 95% confidence intervals (CIs). All variables exhibiting a statistically significant univariate association were integrated into the multivariate model. Differences in OS across groups were appraised utilizing the log-rank test and visually depicted using Kaplan-Meier curves with “ggplot2”[19] and “survminer” packages [20]. A significance level of p ≤ 0.05 was deemed statistically noteworthy.
Clinical Characteristics
The study included 240 patients with OC (n=46), PX (n=75) (oro- PX and hypo-PX) and LC (n=119). Comprehensive details regarding baseline patient and tumor characteristics can be found in (Tables 1-3). Noteworthy risk factors were identified in univariable analysis for the primary endpoint. Overall survival (OS) was incorporated as covariates in multivariable Cox regression models (Tables 1-3). The study group included more men than women (73.3% vs. 26.7%) and the median age was 62 years (range 37 to 97). Tumor volume, as represented by the median GTV-P, spanned from 5 to 196 cm3, with a median value of 29 cm3 across all cases. The median GTV-P values were 37.9cm3 for OC, 41.78cm3 for PX, and 18.84cm3 for LC, respectively based on pretreatment CT scans. The median follow-up time for the entire cohort group was 47 months, with a corresponding median OS of 48 months (median 5YOS 44%). Eighteen patients did not complete the scheduled (C)RT within the designated timeframe due to encountered adverse events: 5 in OC, 6 in PX, and 7 in LC. In the OC group, the use of steroids during RT emerged as the negative prognostic factor, in both univariate and multivariate analyses (p=0.032; p=0.071).
Within the PX group, factors found to have a negative impact on OS in univariate analysis included: diagnosis age over 60 (p=0.02), advance T-stage (T1-2 vs. T3-4; p=0.03), poor performance status (ECOG ≥ 1; p=0.021), unscheduled premature completion of (C)RT (p=0.003), and the necessity to use steroids during RT (p=0.054).
In the LC group, independent factors with negative impact on outcomes included: advanced T-stage (T1-2 vs. T3-4; p=0.050), poor performance status (ECOG ≥ 1; p=0.005), the presence of pathological nodes (N0 vs. N1-3; p=0.003), and the need to use antibiotics during RT (p=0.050). Additionally there was a strong correlation between the total dose of cisplatin administered across all sites (OC, PX, and LC) and long-term outcome. Specifically, this correlation was observed in OC (p=0.010), PX (p=0.034), and LC (p=0.001) in univariate analysis.
Baseline Inflammation Biomarkers and GTV-P
The time-dependent receiver performance characteristics (ROC) curves revealed pretreatment cut-off values for NLR and PLR for each tumor site, for OC 3.89 and 248.08; for PX 1.78 and 155.19, and for LC 2.78 and 227.33, respectively. High-NLR values were identified as independent unfavorable prognostic factors in all tumor sites with statistical significance observed in PX (0.009), and LC (p=0.006) in univariate analysis. High-PLR at baseline was also correlated with poor outcomes in OC (p=0.002), and LX group in univariate and multivariate analysis (p=<0.001; p=0.001) (Table 4). The primary tumor volumes were divided by the median into small and large tumors (≤mGTV-P; >mGTV-P) for OC, PX, and LC, with the median GTV-P (mGTV-P) being 37.9 cm3 in OC, 41.7 cm3 in PX, and 18.8 cm3 in LC. The Kaplan-Meier survival curves for small and large OC, PX, and LC based on baseline NLR and PLR are shown on (Figures 1 & 2). High-NLR and high-PLR were found to be significantly prognostic for poor outcomes in both small (≤mGTV-P) and large tumors (>mGTV-P) across all tree tumor sites. Patients were divided into four groups depending on tumor volume (small vs. large) and NLR/ PLR (low vs. high).
Table 4: Univariate and multivariate analysis of tumor volume (GTV-P), NLR and PLR related to overall survival (OS).
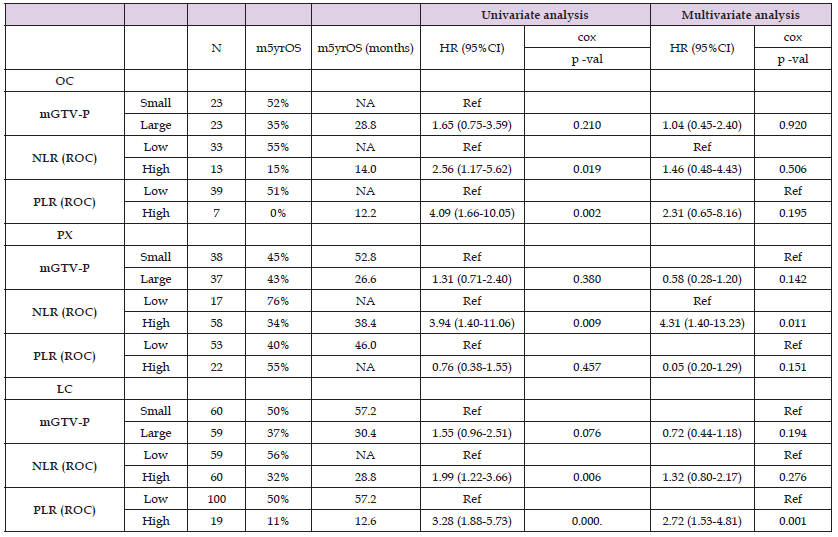
Patients with small tumors and low-NLR had the best long-term outcomes at all sites, with 5YOS for small tumors and low-NLR for OC, PX, and LC being 60%, 76%, and 70%, compared to 23%, 20%, and 25% for small tumors with high-NLR, respectively. Notably, NLR had a strong impact on prognosis among small tumors, with 60% 5YOS in OC for low-NLR compared to 23% for small-high- NLR (Figure 1). In addition, in large tumors, an initial low level of inflammatory markers showed better outcomes comparable to those seen in patients with much smaller primary tumor volumes. High- PLR pretreatment emerged as a strong independent negative factor in treatment results. Patients with small tumors and low-PLR had the best prognosis, whereas patients with high-PLR appeared to have poorer survival, regardless of the volume of primary tumors (Figure 2). The mGTV-P alone did not show significant baseline results for OS in both univariate and multivariate analyses.
The TNM system for tumor staging is widely used to predict oncological treatment outcomes in HNC. The vast majority of failures in radical treatment is attributed to locoregional relapses at or near the original site or in nearby lymph nodes [6,21]. Despite the distinct differences in patient prognosis between stages I to IV, there is currently no consensus regarding the customization of RT doses for the primary tumor and affected lymph nodes based on their volume in HNC cases. Specific prognostic and predictive markers unique to this patient group compared to other types of cancers are still under investigation. However, it has long been recognized that inflammatory markers play an unquestionable role in cancer development, and can influence the course of disease negatively. Our retrospective analysis aimed to investigate the prognostic value of the pretreatment values of NLR and PLR in correlation with tumor volume in patients receiving radical (C)RT for squamous HNC.
To estimate the primary TV (GTV-P), we employed a mathematical approach using the formula for the volume of a sphere, V=4/3 πr3. T1 tumors, with a radius up to r=1cm, had volumes ranging from <0.5cm3 to 4.2cm3. for T2 tumors, with radii from 1cm to 4cm, had volumes ranging from 4.2cm3 to 33.5cm3. T3 tumors with radii over 4cm, had volumes exceeding 33.5cm3. This differentiation in TV within the various subcategories of the T feature alone suggests that the TNM scale may not provide sufficient information for making treatment decisions.
Numerous studies have indicated that calculating TV based on preoperative imaging examinations, can predict the risk of disease recurrence. In many cases, this data proves to be more significant than the TNM classification in guiding treatment decisions and predicting outcomes [21-24]. This crucial aspect is highlighted in the latest guidelines issued by DAHANCA (Danish Head and Neck Cancer Group). The guidelines recommend administering a higher radiation dose of 68 Gy to the GTV-P when its diameter exceeds 4 cm. Conversely, for smaller GTV-P, a lower radiation dose of 66 Gy is recommended. This approach underscores the importance of tailoring radiation therapy doses based on the size of the primary tumor, aligning with the understanding that TV is a significant factor in treatment planning and prognosis for head and neck cancer patients [25]. Flecher, Ferber, and Maciejewski were pioneers in introducing and promoting the concept that the number of cell clones is closely linked to TV, and this relationship has a significant impact on treatment outcomes. Their work contributed to our understanding of how TV plays a crucial role in determining the effectiveness of RT [26-28]. On the contrary, a primary tumor is inherently heterogeneous and does not consist of uniformly distributed tumor cells. Moreover, as the TV increases, its mechanisms of angiogenesis often become insufficient, leading to central hypoxia and subsequent cell death within the tumor.
The relatively high presence of necrotic tissue within large primary tumors is undeniably one of the contributing factors to the high rate of local failures. Clusters of hypoxic, necrotic cells are inherently resistant to (C)RT. We regularly observe these fundamental principles of oncology, for instance through the visualization of HNC using 18-fluorodeoxyglucose (FDG)-positron emission tomography / computed tomography (PET / CT). Beat Bojaxhiu and colleagues conducted an analysis of NLR/PLR and metabolic parameters in oro-PX cancer patients. They found that the maximum standardized uptake value (SUV) did not exhibit a correlation with NLR levels or PLR. However, they did observe a correlation between PLR and metabolic tumor volume (MTV) (p = .03) as well as total lesion glycolysis (TLG) (P = .02). Apart from TLG, which was associated with worse survival in both univariate and multivariate analyses, no other metabolic PET/CT parameters were found to be linked to either OS or disease-specific survival [29]. This underscores the complex interplay between tumor heterogeneity, metabolic parameters, and treatment outcomes in HNC cases. Recently, de Andrade et al. found that TV has an impact on a higher risk of 5-year disease-free survival (DFS) and worse overall survival (OS) in univariate analysis. However, these findings were not confirmed in multivariate analysis. This suggests that while TV may be a relevant factor in predicting outcomes, it may not be the sole determinant, and other factors may play a role.
Additionally, the significance of TV can vary between different studies and patient populations [30]. In our study, TV alone did not exhibit statistical significance for OC, PX, and LC, similar to the findings in some other studies. However, it is worth noting that the median TV in our LC group was higher compared to the OC and PX groups, which could potentially impact the results. A study by Adrian et al. in 2022 also explored the importance of TV in oropharyngeal squamous cell carcinoma. In a group of over 500, patients they found that the impact of TV on prognosis was less pronounced in p16 (-) compared to p16 (+) tumors [21]. This suggests that the role of TV may be influenced by other factors, such as the presence of the p16 protein. In our study, we focused specifically on patients with HPV-negative HNC tumors, which could explain why TV alone did not have a clear impact on OS. The interaction between various factors, including tumor characteristics, HPV status, and inflammatory markers like NLR and PLR, can be complex and may influence treatment outcomes differently. Similar results were presented by Ahlawat and colleagues showing that the adjusted hazard ratio for OS per 1 cm3 increase in TV was 2.3% for p16-positive and 1.3% for p16-negative tumors [31]. We divided all patients into four groups depending on GTV-P (small vs. large) and NLR/PLR (low vs. high). In the analysis of these two variables, we showed that for all three HNC locations, high-NLR and high-PLR were the worst prognostic factors. Patients with small-high- NLR/PLR tumors had a much worse prognosis compared to patients with small-low-NLR/PLR tumors (5YOS, 23% vs. 60%).
Surprisingly, patients in the large-low-NLR/PLR group did not show much worse long-term outcomes compared to the small-high- NLR/PLR group. Overall, readily available markers of inflammation, like different peripheral blood cell (CBC) groups, and indirect measurements such as malnutrition indicators, have been the subject of significant research interest. Exploring these markers in various cancer types can help clinicians tailor treatment strategies more effectively and potentially identify patients who may benefit from additional interventions to mitigate inflammation-related risks. Further studies in this area are essential to improving our understanding of the role of inflammation in cancer and its impact on patient outcomes across different tumor types [32-35]. In a 2021 meta-analysis, Kumarasamy C, et al summarized the results from 49 publications on the role of NLR, PLR and monocyte–lymphocyte ratio (MLR). The pooled HR values of PLR, NLR and MLR indicated clear significant correlation with worse OS. The pooled effect estimates for PLR, NLR and MLR were 1.461 (95% CI 1.329–1.674), 1.639 (95% CI 1.429–1.880) and 1.002 (95% CI 0.720–1.396), respectively. The authors extensively discuss and emphasize the usefulness of the PLR and NLR ratios, while pointing out the uselessness of the MLR. However, they did not analyze the cut-off points for individual variables [36]. There is an ongoing challenge of determining cutoff values for individual parameters, such as NLR and PLR, in predicting treatment outcomes for HNC patients.
Our study showed that the pretreatment cut-off point for NLR (2.8) was clinically significant, while PLR (130) was not clinically significant in patients with LC managed with definitive RT or CRT. We propose a reliable cutoff for NLR in HNC to be around 2 to 4, with potential variations based on specific site of the tumor. The cutoff for PLR should be relatively high, in the range of 155 to 250 to possess predictive power. In our study, the PLR cutoff of 227 for LC was CS, in both univariate and multivariate analyses. It should be emphasized that further validation through a large prospective multicenter study would be necessary to firmly establish these cutoff points. The identification of distinct patient groups, such as those with large tumors and low-NLR/PLR, and those with small tumors and high pretreatment NLR/PLR, underscores the complexity of HNC prognosis and the need for additional risk stratification criteria to tailor RT and CRT schedules effectively. It is important to acknowledge the limitations of our retrospective study. Although all patients had consistent radiotherapy CT planning imaging, any missing data, can affect the quality of the dataset. Additionally, some patients required TNM reassessment according to the 8th edition, and P-16 positive patients were excluded, which may impact the generalizability of the findings. Lastly, the lack of data on disease-free survival and patterns of relapse highlights the need for comprehensive follow-up studies to gain a more comprehensive understanding of treatment outcomes in HNC patients.
Peripheral immune markers, particularly high-NLR and high-PLR significantly impact the outcomes of patients with OC, PX, and LC who receive definitive RT or CRT. Our findings highlight the importance of incorporating these markers into pre-treatment risk stratification methods, particularly for small volume tumors. By recognizing the prognostic value of NLR and PLR, healthcare providers can better identify patients who may be at higher risk for adverse treatment outcomes and may benefit from tailored interventions or treatment adjustments. This insight emphasizes the significance of immune markers in refining treatment strategies and ultimately improving the prognosis and quality of care for individuals with OC, PX, and LC cancer.
The authors declare no conflict of interest.
We would like to thank the staff who took care of our patients’ needs, and who were involved in gathering, documenting, verifying, forwarding and processing the clinical data.
This study was approved by the Bioethical Committee of the Medical University of Gdańsk (NKBBN/357-298/2016) and was carried out in accordance with the Declaration of Helsinki, and the requirement for informed consent was waived because of the retrospective design.


