Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Daniel Y. Reuben1* and Andraia R Li2
Received: July 18, 2023; Published: September 26, 2023
*Corresponding author: Daniel Y. Reuben, Department of Medicine, Medical University of South Carolina, 173 Ashley Ave., MSC#635, Charleston, SC, USA 29425
ORCID ID: 0000-0003-3593-4690
DOI: 10.26717/BJSTR.2023.53.008338
Background: Responses to immune checkpoint inhibitor (ICI) therapy is variable with regards to response type and duration with patients with melanoma. To date an arbitrary treatment duration is applied in absence of clear biomarkers which could otherwise guide therapy. Eosinophilia has recently been investigated to assess its utility as a biomarker with respect to ICI use in this disease.
Objective: To compare responses to immunotherapy with developing serologic eosinophilia and relate to patient demographics, stage of disease and response attained.
Methods: A single-institution retrospective study of 114 patients with advanced melanoma described by treatment type, disease stage and duration of treatment was performed. Chart review was undertaken and assessment of serologies, time to eosinophilia, if it occurred, correlation with response and stage of disease was investigated. Statistical analysis was performed to assess for significance at p<.05.
Results: Of the 114 identified patients, 99 (86.8%) had undergone treatment with ICIs of which 32.3% would also experience eosinophilia. The mean time to eosinophilia was 4.2 months among these patients and the average peak absolute eosinophil count (AEC) was 0.86 K/mm3. In patients who experienced eosinophilia a greater number of complete responses were seen (68.8% vs 59.7%). Eosinophilia was associated with a trend in improved survival (Hazard ratio = 0.38, 95% Confidence Interval 0.74-1.99), but non-statistically significant in this short follow-up period. There were only modest differences in early development (<6 months) versus late development (≥6 months) of eosinophilia and not found to be clearly correlated to the response type. Early development of eosinophilia was seen with ipilimumab. A weak correlation of delayed eosinophilia with advanced age was seen.
Conclusion: Eosinophilia development occurred with numerous types of immunotherapies. When seen with ipilimumab this developed earlier with statistical significance. No difference in eosinophilia development correlated with age of patients was seen. A weak statistical correlation with delayed eosinophilia with advanced age was found. ANOVA showed a statistical correlation of eosinophilia with response. A trend to improved O.S. was seen although not statistically significant in the short time of follow-up herein. Eosinophilia may have a contribution to predicting ICI response in this patient population but further study will be required to assess long-term outcomes such as survival.
Keywords: Melanoma; Immunotherapy; Checkpoint; Eosinophilia; Eosinophils; Biomarker
Abbreviations: ICI: Immune Checkpoint Inhibitor; CR: Complete Response; PR: Partial Response; SR: Stable Response; OS: Overall Survival; ANOVA: Analysis of Variance; TME: Tumor Microenvironment; MUSC: Medical University of South Carolina; PET: Positron Emission Tomography
Treatment of melanoma with systemic therapies has recently evolved. The prior use of chemotherapy has been associated with poor response rates in the treatment of advanced disease [1]. However, the introduction of immune checkpoint inhibitors (ICI)s has heralded a new manner in which many patients with cancer are now treated. ICI therapy was established in the 1990s and ICIs entered clinical trials for metastatic melanoma patients with resulting high response rates in a small series in the early 2000s [2,3]. Patients with metastatic melanoma were among the first successfully treated with this approved immunomodulatory approach. Numerous other cancers are now found to be responsive to ICIs as well [4,5]. Response to ICIs became better understood with successive large trials showing a spectrum of non-responders, partial responders and those with a complete response, potentially durable [6,7]. Despite these advances, it remains to be seen what prognostic factors or features portend a response to immunotherapy. Given this, patients have been treated with immune-modulators and ICIs with heterogenous outcomes [8-10]. Similar to forecasting the efficacy of a particular chemotherapy for treatment of other cancers, the effectiveness of ICIs in the treatment of melanoma has been difficult to predict for specific patients [11]. Demographic features such as age, sex, BMI, stage and performance status have been investigated. Of these only performance status and organ involvement are correlated retrospectively to improved overall survival (OS) and therefore responses [12]. Poorer survivorship has been linked to sex, site of disease and staging but translating this into treatment decision making has not yet occurred [13]. Other strategies such as investigating the tumor microenvironment (TME) with regard to specific populations of immune cells and cell marker expression has been undertaken [14]. Drawbacks to this strategy include the heterogeneity of the TME among different metastatic lesions, the difficulty of obtaining sufficient biopsies, standardization, availability and timeliness of such assays [15]. This leads to the consideration of a useful and easily available serologic study. Along this line investigators have looked at eosinophilia.
Beyond its role as an intermediary in facilitating allergy and inflammatory responses, there is growing evidence to suggest eosinophils are important effectors of the TME [16]. Eosinophils release directly cytotoxic proteins and granules including eosinophil cation protein, classically known for their helmintholytic activity, and have been shown to be tumoricidal as well [17]. Preclinical evidence has implicated eosinophils in the recruitment of cytotoxic CD8+ T cells, increasing T-cell infiltration and subsequently augmenting tumor rejection [18]. Additionally, eosinophils have also been shown to stabilize vascularization, decreasing vessel leakiness and perfusion in addition to macrophage polarization to the neurotoxic M1-population [18]. It is this coordinated relationship of eosinophils and the TME that is thought to underpin the synergistic role for ICI use in cancer therapy and why eosinophilia may be a positive prognosticator of ICI response in melanoma patients [15]. To date, the association of eosinophils with cancer treatment continues to be complex. Elevated levels of serum eosinophils have been found and shown to be a favorable prognosticator for survival in melanoma [19]. breast cancer [20] and colorectal cancer [21] but unfavorable in cervical [22] and squamous-cell lung cancer [23,24]. Due to the lack of clarity further studies correlating eosinophil levels and effector function with ICI use and type of disease are needed. Identifying a prognostic signal of ICI response would be most helpful ahead of or early into treatment as a signal would allow for the treatment to be tailored to alternate strategies if not effective. Also, it may aid reassurance to patients with ICI toxicity requiring a treatment holiday. With consensus being a one- or two-year ICI treatment period for stage III and IV melanoma respectively, the ability to modify treatment duration based on an individual patient’s response would be helpful to reduce risks and costs associated arbitrary lengths of treatment. Here, we report a single institutional retrospective experience of eosinophilia with correlation of treatment type, staging, time to onset and correlation with demographics.
The procedures and protocols of the study were approved by the Medical University of South Carolina (MUSC) Institutional Review Board (Protocol #00115093). We retrospectively reviewed the consecutive charts of patients seen for treatment or follow-up with advanced stage melanoma over a one year period (September 30, 2020 to October 1, 2021) by a single clinic in follow-up at MUSC. In total, 122 patients with advanced melanoma were identified. The patients’ medical records were reviewed to obtain relevant demographics including age, stage, treatment type, lines of therapy received, response and survivorship as measured by overall survival (OS). An inventory of CBC and differential counts were reviewed to identify a maximum absolute eosinophil count (AEC), where an AEC >0.50 K/mm3 was defined as eosinophilia, and correlated with treatment to assess time to eosinophilia, if it occurred, and treatment response. Patients without clearly documented staging, assessed eosinophil levels or documentation of response were excluded from analysis. Deidentified raw data was collected as per local IRB regulations. A response was assessed using Positron Emission Tomography (PET) Response Criteria in Solid Tumors (PERCIST) 1.0 criteria. A complete response (CR) was defined as complete resolution of 18F-fluorodeoxyglucose (FDG) uptake on PET within the target lesion(s). A partial response (PR) was demonstrated when greater than 30% decrease in target tumor FDG uptake occurred. A stable response (SR) was judged when neither a partial response, complete response (CR) or progressive disease (PD) is observed. Progressive disease is defined as greater than 30% increase in FDG uptake or presence of a new FDG positive lesion [25]. The Fisher exact test and T-test were used for statistical analysis with a p-value less than 0.05 considered statistically significant. A Kaplan-Meier survival analysis was computed and graphed with GraphPad Prism version 8.0 (San Diego, California, US). Spearman correlations, Chi-Squared regression analysis, ANOVA and graphical representation of this data was performed using DATAtab Online Statistics Calculator (Graz, Austria).
Sequentially identified patients with melanoma seen in the follow-up period numbered 122. A total of 8 patients were excluded for the following: unknown response (N=6), no documented stage of disease (N=1) and no eosinophil levels assessable (N=1) (Figure 1). A total of 114 patients, with a median age of 69 years old, were represented in our analysis. Of these patients, 43.9% and 54.4% were diagnosed with stage 3 and stage 4 disease, respectively. A large number of patients were older adults. A histogram depicts the population studied with respect to age rounded to half-deciles (Figure 2). The majority of patients, 86.8% (N=99) had undertaken ICI therapy while 13.2% of patients (N=15) were not treated with ICIs. This included 9 patients who did not undergo any treatment secondary to preference or potentially having a contraindication. In 32.5% of cases, immunotherapy was administered in addition to other modalities of therapy: surgery (beyond initial resection), radiation and/or chemotherapy. Of the patients treated with ICIs, treatment included the following: 62 patients were treated with pembrolizumab; 22 were treated with nivolumab; 14 were treated with ipilimumab, 33 were treated with combination ipilimumab/nivolumab and one remained blinded from the Checkmate 238 trial [26] (totals exceed n=99/100% as multiple lines of therapy were given in some cases) (Table 1).
Overall responses were tabulated. In the patient population studied (N=114), 76 attained and maintained a CR, 14 a PR, 3 SR, and 21 showed progression of disease. When investigating responses with respect to age there was not a statistical difference at p < .05 among the 4 response groups by ANOVA F (3,110) = 0.91, p = .439, ɳ² = 0.02) (figure 3). A total of 32 patients in this study demonstrated eosinophilia amidst the follow-up period. Of the 99 patients treated with ICIs, 31.3% (n=29) experienced eosinophilia while 13.3% (N=3) of 15 patients who did not undergo immune checkpoint inhibition experienced eosinophilia. The mean peak AEC was 0.84 K/mm3. The proportion of patients with eosinophilia from any cause in stage 3 disease was 10.5% (N=12) and stage 4 disease was 17.5% (N=20). There were differences in the rates of CR (68.8% vs. 59.7%), PR (9.4% vs. 14.9%), SR (6.3% vs. 1.5%) and progression (15.6% vs. 23.9%) between patients who did (N=32) and patients who did not (N=67) experience eosinophilia (Table 2). While a CR was attained in many instances without any development of eosinophilia it appeared that the converse was also true: developing eosinophilia lent a good chance of future CR. Only in 5 cases of developing eosinophilia was there progression of disease (Figure 4). Logistic regression analysis was performed to examine the influence of eosinophilia on best response to predict CR. This analysis showed that the relationship of eosinophilia and CR was not significant χ²(1, N=114) = 0.09, p = .767. No significant associations with the other response groups were seen either at p < .05.
Table 2: Responses are tabulated and compared to patients with regards to eosinophilia, if it developed, and by time to eosinophilia.
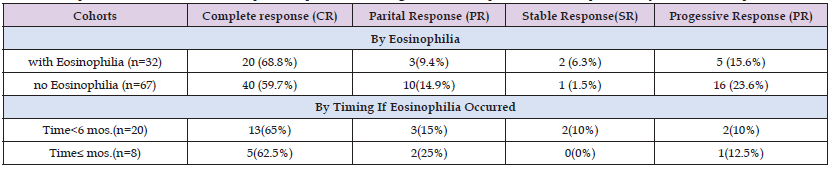
Further characteristics of patients with eosinophilia were investigated in the data set. This included timing of eosinophilia. In 65.6% (N=21) of patients with eosinophilia this occurred during the first line of treatment. Time to eosinophilia following initiation of treatment ranged from 1 month to 19 months with a mean time from treatment start of 4.2 months. A large variability to time of onset was seen. In investigating any relationship to the specific ICI therapy and time to eosinophilia a Spearman correlation was undertaken. The result showed that there was a significant correlation between what treatment was given and time to eosinophilia in months, r(112) = 0.97, p = <.001. Principally ipilimumab appeared to precipitate eosinophilia sooner and with consistency than anti-PD1 agents (Figure 5). Time to eosinophilia on basis of stage of disease was also investigated. Using a Spearman correlation, there was no significant correlation between stage and time to eosinophilia, r(110) = 0.12, p = .197 (Figure 6). Owing to some patients having delayed eosinophilia a 6-month timepoint was investigated. Of the 29 patients demonstrating eosinophilia associated with ICI therapy, this occurred at least once before 6 months in 22 patients and ≥ 6 months in 7 patients. There was no significant difference in rates of response between patients with eosinophilia occurring <6 months following initiation of treatment and patients with time to eosinophilia ≥6 months, individually by Fisher’s exact test (p > .05), (Table 2).
However using ANOVA results were significant at a p < .05 with respect to the SR group: F(3,110) = 4.7, p = .004, ɳ² = 0.11. In subtracting patients with SR, as there were only 3 datapoints in this category, ANOVA did not show a statistical difference in overall responses at any time with regards to eosinophilia development F(2,108) = 0.6, p = .551, ɳ² = 0.02 (Figure 7). As changes in the immune response can occur with age it was investigated if a difference in patient age with respect to the cohort that exhibited eosinophilia could be detected. A Spearman correlation was performed to determine if there was a correlation between the time to eosinophilia (if it occurred) and patient age. This showed that there was a low, positive correlation between the variables with r(112) = 0.22, p = .02. Thus, a small, positive association between eosinophilia and advancing age was demonstrated with a modest delay in eosinophilia developing in older patients (Figure 8). Median duration of OS was 48.5 months in the eosinophilia cohort and 27.0 months in the no eosinophilia cohort at time of analysis albeit endpoints were not reached in 93.0% of patients. There was no significant difference in the estimated hazard ratio for death (0.38; 95% confidence interval (CI)(0.74-1.99)) between both cohorts although a trend is noted (Figure 9).
Laboratory studies are routinely monitored prior and throughout immunotherapy treatment. Early reports suggest there may be an association between improved survival and lymphocyte count [27]. More recently, assessing eosinophil levels as a potential predictor for ICI response has been emphasized [28]. We show a trend in OS outcomes depending on the presence of eosinophilia as measured by AEC coinciding with initiation of ICI therapy. Our data also shows an increased number of CR responses and decreased number of progressive disease responses in the cohort who manifested eosinophilia although a statistical significance was not reached in our sample. With a larger effect and longer time in follow-up potentially a difference in O.S. could be seen. Supporting this, improved overall survival with eosinophilia was found in a similarly-sized cohort study of metastatic melanoma patients over 12 years where eosinophilia was associated with a mean improvement in survival of 19 months in response to ICI therapy (so long as survival initially exceeded one year for such patients) [19]. Time to eosinophilia on ICI therapy has been investigated and may also provide prognostication. We also reviewed this with respect to those patients who demonstrated eosinophilia. Since most eosinophilia was seen early (<1 year from treatment start), we analyzed whether eosinophilia before or after 6 months’ treatment were associated with any differences in outcome. In our cohort having a relatively short follow-up no statistical difference in survival outcomes were seen with respect to this factor. This contrasts with another report whereby late development of eosinophilia greater than one year from treatment start was associated with an increased mean OS of 7.8 months over patients who had earlier manifestation of eosinophilia (29). A small retrospective study however showed that ICI responders manifest eosinophilia in as early as 6 weeks, albeit without statistical significance in relative eosinophil levels between responders and non-responders (p=.10) [30].
In contrast, in a study of 59 patients with metastatic melanoma treated with ipilimumab, eosinophilia occurred early in treatment, often after a single dose of ICI therapy, and was significantly associated with an improved clinical outcome (p<.001) [31]. Given these varied reports drawing definitive conclusions regarding significance around the timing of eosinophilia is difficult. Potentially it may depend on the type of ICI used, line of therapy, stage of disease or patient factors not recorded. We do show a similar and statistically significant difference in time to eosinophilia based on type of ICI used. We also show that ipilimumab promoted eosinophilia sooner than patients treated with anti-PD1 therapies when it occurred. We quantified eosinophilia using the AEC. The rationale is that this metric is not dependent on other cells in the differential count which can occur when choosing relative percent levels. As many patients can manifest relative neutrophilia or other shifts in the differential count due to undisclosed rheumatic, vascular, infectious or genetic factors, choosing an absolute eosinophil count was felt to allow less confounding in attempting to correlate with ICI use [32]. Discrepant results among published studies may also be from this difference in how eosinophilia is measured.
This report is also limited as a construct with retrospective, single-center and single provider data. Ideally assessing eosinophilia in prospective form may help to ascertain its significance more clearly. Larger endeavors to associate many serum results in combination with stage and location of metastases are notable but have not yet been adopted in clinical care [33]. This points to a consideration that a single factor such as eosinophilia may have a relative weak contribution to prognosis. With mean time to eosinophilia observed at 4.2 months as shown in our study, patients will have a quarter of their course of treatment, depending on their disease stage, prior to developing a clear response type. However, tying this to other factors, if they exist, may both strengthen the prognostic signal but also confound easy applicability if many factors are involved and should they have various weighting. In summary, we show improved responses with increased numbers of CR and decreased progressive events with melanoma patients who manifest eosinophilia amidst ICI therapy. A non-statistical trend to improved survival outcomes, when using ICI therapy with the demonstration of peripheral blood eosinophilia, was seen with short follow-up. Eosinophilia developed statistically earlier with the use of ipilimumab. Older patients manifest eosinophilia with modest delay compared with younger patients. Eosinophilia, if it occurred, did not appear to depend on stage of disease. Further long-term analysis will be needed to determine statistical significance of survival in this study. Presently this helps to expand a growing body of research on eosinophilia and cancer treatment. The correlation of eosinophilia and outcomes is still unclear and contrasting results with other reports is presented. Future incorporation of biomarkers such as eosinophilia in newly designed trials may provide further prognostic data for patients. This feature may be important for clinicians to allow adjustment of the duration and type of ICI treatment for selected patients.
None.
The authors declare no conflict of interest.
Author T assisted with conceptualization, data collection, statistical analysis, manuscript writing and editing. Author AL provided data review, statistical analysis, and manuscript writing.


