Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Yuko Naya1, Kikumi Ogihara1 and Takashi Kuribayashi2*
Received: August 17, 2023; Published: September 08, 2023
*Corresponding author: Takashi Kuribayashi, Laboratory of Immunology, School of Life and Environmental Science, Azabu University, Kanagawa, 1-17-71 Fuchinobe, Chou-ku, Sagamihara, Kanagawa 252-5201, Japan
DOI: 10.26717/BJSTR.2023.52.008301
The kinetics of α2-macroglobulin (α2M) were evaluated in rats with acute severe renal failure. This renal failure was induced by repeated subcutaneous injections of gentamicin at a dose of 80 or 120 mg/kg for 10 days. Twenty-two male Sprague-Dawley rats were divided into two groups for two experiments. Turpentine oil was intramuscularly injected at a dosage of 0.2 mL/kg body weight at 2 days after finishing the injections of gentamicin. The serum concentrations of α2M were then measured by enzyme-linked immunosorbent assay. At 5 and 10 days post-injection, both the creatinine (Cr) and neutrophil gelatinaseassociated lipocalin (NGAL) values were significantly higher in the 120-mg/kg gentamicin group than in the control group. However, significant differences in blood urea nitrogen (BUN) were observed only at 10 days in both the 80 and 120-mg/kg gentamicin groups. The most pronounced histological changes were seen in a rat of the 120-mg/kg group; this rat showed the highest levels of BUN, Cr and NGAL. The maximum serum concentration (Cmax) and area under the blood concentration vs. time curve (AUC) were significantly higher in the 80-mg/kg gentamicin group than in the control group. The serum concentrations of α2M were significantly higher in the 80-mg/kg gentamicin group than in the control group; however, the half-life did not significantly differ between the gentamicin-treated and control groups. Conversely, no significant differences were observed in the serum concentrations of α2M or the Cmax and AUC between the 120-mg/kg gentamicin and control groups. We found that although the serum level of α2M increased in cases of renal failure, it did not show a similar increase in cases of severe acute renal failure.
Keywords: α2-Macroglobulin; Acute Severe Renal Failure; Gentamicin; Neutrophil Gelatinase-Associated Lipocalin; Rat
α2-Macroglobulin (α2M) is a broad-spectrum protease inhibitor that has seven members in humans and two members in mice according to MEROPS, the peptidase database [1-4]. α2M is also recognized as a typical acute-phase protein and a useful inflammatory marker, since the serum concentration of α2M increases markedly during acute inflammation in rats [5-7]. The serum concentrations of α2M have been reported to decrease in rats with induced hepatic failure [8,9]. In contrast, the serum concentrations of α2M reportedly increase in humans with renal impairment [10,11]. Similarly, serum concentrations of α2M also increased in rats with induced renal failure through intravenous injection of gentamicin (GM) at a dose of 50 mg/kg [12]. However, the half-life (t1/2) of α2M remains unchanged in these rats [12]. It is unclear why the kinetic parameters of α2M do not change in rats, but it is thought to be related to the severity of renal failure. GM, an aminoglycoside antibiotic, is known to be nephrotoxic and to cause tubule damage [13-17]. Intravenous injection of GM at more than 50 mg/kg resulted in rat fatalities, making it impossible to induce severe acute renal failure with an increased dose via this administration route. As such, the method of administration needed to be changed to subcutaneous injections of GM to induce more severe renal failure, as an increased dosage via this route does not result in rat fatality. The purpose of the present study was to clarify the kinetics of α2M as an acute-phase protein in rats with acute renal injury of greater severity.
Animals
In this study, 22 male Sprague-Dawley rats (weight: 180 – 270 g, age: 6 weeks old) were purchased from CLEA Japan (Tokyo, Japan), and divided into two groups for two experiments. The first experiment used 12 rats: 7 rats were injected with 80 mg/kg of GM, and 5 rats were used as controls. The other experiment used 10 rats: 5 rats were injected with 120 mg/kg of GM, and 5 rats were used as controls. Rats were housed individually at a temperature of 230C ± 20C and a relative humidity of 55% ± 10% on a 12-h dark (20:00 to 8:00) and 12-h light (8:00 to 20:00) cycle. The air was exchanged 12 or more times per hour. Rats were fed standard chow (MFTM, Oriental Yeast Co., Ltd., Tokyo, Japan), and allowed free access to water.
Animal Experiments
Two animal experiments were performed. Nephropathy was induced by repeated subcutaneous injections of GM sulfate (Nichi-Iko Pharmaceutical Co., Ltd., Toyama, Japan) once daily for 10 days at a dose of 80 mg/kg (80-mg/kg group) in one experiment, and the same was done at a dose of 120 mg/kg (120-mg/kg group) in the other experiment. The rats in the control group for each experiment were similarly injected subcutaneously with sterile saline. Turpentine oil (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) was used to induce acute inflammation [18]. The turpentine oil was intramuscularly injected at a dose of 2 mL/kg body weight 2 days after finishing the GM injections. Blood was collected before turpentine oil injection, and at 24, 48, 72, 96, 144, 192, 240, 312, 384, and 480 h post-injection. The blood was collected from the jugular vein under mild anesthesia by inhalation of isoflurane (FUJIFILM Wako Pure Chemical Corporation). Serum was obtained by centrifugation at 3,500 rpm for 15 min. Approximately 120 µL of each of the serum samples was stored in freezing tubes at -800C until the α2M and NGAL measurements.
Measurement of α2M and NGAL
The serum concentrations of α2M were measured by an enzyme-linked immunosorbent assay (ELISA) according to the procedure of Honjo et al., with modifications [19]. Serum levels of NGAL were measured by ELISA using a commercial kit (BioPorto Diagnostics A/S, Hellerup, Denmark).
Measurement of Biochemical Parameters
The measurement of the biochemical parameters was outsourced to Hoken Kagaku Co., Ltd. (Kanagawa, Japan). Total protein (TP) was analyzed using the biuret method, BUN was analyzed using the urease-glutamate dehydrogenase method (ammonia removal), and Cr was analyzed using an enzymatic method using an automated analyzer (JCA-BM8060; JEOL Ltd., Tokyo, Japan).
Histopathological Evaluation of the Kidneys
After the final blood sampling, rats were sacrificed under deep anesthesia by inhalation of isoflurane (FUJIFILM Wako Pure Chemical Corporation), and the left kidney of each rat was collected for histological evaluation. Each kidney was fixed in 10% buffered formalin, embedded in paraffin, and sectioned to a thickness of 4 µm. To determine the degree of renal damage in each rat, the number of cells with histological changes in the renal cortex was counted, and the average value from 10 fields of view per sample was scored.
Statistical Analysis
Data were analyzed using GraphPad Prism 7.0 software (GraphPad, La Jolla, CA, USA). All values are expressed as the means ± standard deviation. The maximum serum concentration (Cmax) was determined for each individual rat. The area under the blood concentration vs. time curve (AUC) from 0 to 480 h was calculated using the trapezoidal rule [20]. The linear slope of the serum α2M concentration vs. time was then plotted from the log-linear regression for individual animals [20]. The elimination rate constant (K) was calculated using a minimum of three measured serum concentrations. The terminal elimination half-life time (t1/2) was calculated as ln 2/K [18]. Variations in serum levels and kinetic parameters of α2M, NGAL, BUN, Cr, and TP were assessed using the Mann-Whitney U test. P-values <0.05 were considered to indicate statistical significance.
The body weight and the serum levels of BUN, Cr, TP, and NGAL in the 80-mg/kg, 120-mg/kg, and control groups are shown in (Table 1). In the 80-mg/kg group, only Cr was significantly higher when compared to the control group at 5 days after dosing, and BUN, Cr, and NGAL were significantly higher when compared to the control group at 10 days after dosing. Similarly, in the 120-mg/kg group, only Cr at 5 days, and Cr, BUN, and NGAL at 10 days after dosing were significantly higher when compared to the control group. No significant changes in body weight were observed at 5 days after dosing in both the 80-mg/kg and 120-mg/kg groups; however, at 10 days, the body weight in both groups was significantly lower than that of the control groups. No significant change was observed in TP in both the 80-mg/kg and 120-mg/kg groups when compared to the control groups. (Figure 1) shows typical histological findings from the 80-mg/kg, 120-mg/kg, and control groups. The histological findings from the 80-mg/kg and 120-mg/kg groups included tubular dilatation, flattening, and vacuolation of the tubular epithelium, loss of the proximal tubular brush border, formation of tubular casts, and mononuclear cell infiltration in the stroma. (Table 2) shows the scoring results for the histological changes. The mean scores for the 80-mg/kg and 120-mg/kg groups were 0.83 and 2.19 for tubular dilatation, 0.23 and 1.36 for brush border shedding, 0.20 and 1.01 for tubular epithelial shedding, and 0.10 and 0.73 for hyaline substance, respectively.
Table 1: Body weight and blood biochemistry values of rats injected with repeated subcutaneous doses of gentamicin (GM) at 80 mg/kg or 120 mg/kg for 10 days.
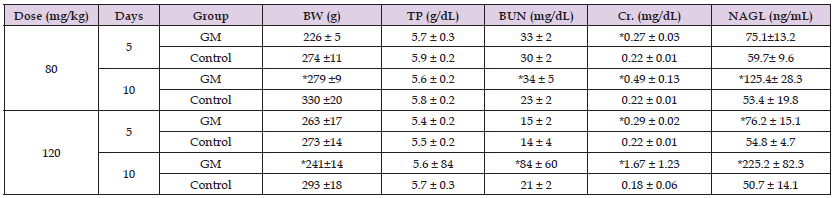
Note: The data represent the mean ± standard deviation. Asterisks indicate statistical significance in comparison to the control group (p < 0.05, Mann-Whitney U test). Days: days after injection of GM, BW: body weight, TP: total protein, BUN: blood urea nitrogen, Cr. creatinine, NGAL: neutrophil gelatinase- associated lipocalin
Figure 1 A. Hematoxylin-eosin staining of kidney samples from rats after the subcutaneous injection at a dose of 80 mg/kg B. And the corresponding controls C. And after the injection of gentamicin at a dose of 120 mg/kg D. And the corresponding controls #: Flattening of the tubular epithelium, *: Proximal tubule brush border loss, l>: Calcification.

Table 2: Mean scores of the histological changes in the renal cortex of rats with severe renal failure induced by repeated subcutaneous injections of gentamicin (GM) at a dose of 80 mg/kg or 120 mg/kg for 10 days.
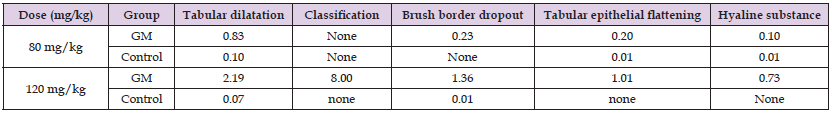
Note: The data represent the mean score. To determine the degree of renal damage in each rat, the number of cells with histological changes in the renal cortex was counted, and the mean value of 10 fields per sample was scored. None indicates that no histologically altered cells were observed in the field of view.
The most remarkable histological findings were observed in a rat with the highest levels of BUN, Cr, and NGAL in the 120-mg/kg group. The changes in the serum levels of α2M in the 80-mg/kg and 120-mg/kg groups are shown in Figure 2. The kinetic parameters in both the 80-mg/kg and 120-mg/kg groups are shown in Table 3. The serum concentrations of α2M in the 80-mg/kg group were significantly higher than those in the control group at 72 h (p = 0.0087), 96 h (p = 0.0087), and 192 h (p = 0.0173) after the injection of turpentine oil (Figure 2). In contrast, no significant difference was observed between the 120-mg/kg and control groups, except for before the injection of turpentine oil (p = 0.0317). Furthermore, to confirm the differences in the α2M concentrations, we considered that a comparison of the AUC and Cmax would be more appropriate than a comparison of serum concentrations at each time point, so we compared the AUC and Cmax to those of the control groups. The AUC of the 80-mg/kg group was significantly higher than that of the control group (Table 3). However, no significant differences in the Cmax or AUC were observed between the 120-mg/kg and control groups. In addition, no significant differences in the K and t1/2 were observed between both GM injection groups and the control groups.
Figure 2 Serum levels of α2-macroglobulin (α2M) in rats with severe renal failure induced by repeated subcutaneous injections of gentamicin (GM) at a dose of 80 mg/kg (A) or 120 mg/kg (B). Turpentine oil was intramuscularly injected at 0.2 mL/kg body weight to induce acute inflammation 2 days after finishing the injections of GM. The data represent the mean ± standard deviation. Asterisks indicate statistical significance in comparison to the control group (p < 0.05, Mann-Whitney U test). Serum concentrations of α2M were significantly higher in the 80-mg/kg GM group than in the control group at 72, 96, and 192 h after turpentine oil injection, and were significantly higher in the 120-mg/kg GM group than in the control group before turpentine oil injection.

Table 3: Kinetic parameters of α2-macroglobulin in rats with severe renal failure induced by repeated subcutaneous injections of gentamicin (GM) at a dose of 80 mg/kg or 120 mg/kg for 10 days.
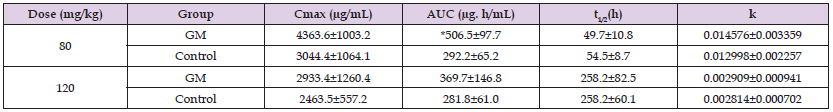
Note: The data represent the mean ± standard deviation. Turpentine oil was intramuscularly injected at 0.2 mL/kg body weight to induce acute inflammation 2 days after finishing the injections of GM. The asterisk indicates statistical significance in comparison to the control group (p < 0.05, Mann-Whitney U test). Cmax: maximum concentration, AUC: area under the blood levels vs. time curve, t1/2: half-life
The kinetics of α2M were evaluated using rats in which severe renal failure was induced by the injection of repeated intraperitoneal doses of GM at 80 mg/kg or 120 mg/kg. The doses of GM used for inducing renal failure in rats in the present study were determined in a preliminary study. Rats were injected intraperitoneally with GM at a dose of 120 mg/kg or 150 mg/kg, and all of the rats injected at the dose of 150 mg/kg died due to severe toxicity within a few days after injection (data not shown). Thus, this study was conducted with the doses of 80 mg/kg and 120 mg/kg of GM. The production of NGAL increases in the distal tubule as a result of tubular damage, and the NGAL inhibits reabsorption from the proximal tubule, resulting in increased urinary excretion [21-22]. However, accurate evaluation of renal damage from the urinary NGAL concentration requires urine collection and special laboratory equipment, e.g., a urinal. Therefore, in this experiment, we examined whether evaluation of renal damage using the serum NGAL concentration is possible as an easier method. At 5 days after injection, there was no significant difference in the serum level of NGAL between the 80-mg/kg and control groups, while the level of Cr was significantly higher in the 80-mg/kg group than in the control group. Since NGAL was found to be an earlier marker of renal damage than Cr or BUN, it is considered a useful index of acute kidney injury [21,22]. In the present study, the NGAL concentration tended to be higher after 5 days of GM administration in the 80-mg/kg group when compared to the control group (p = 0.0631).
The serum levels of NGAL, BUN, and Cr at 10 days after GM injection were significantly higher in the 80-mg/kg and 120-mg/kg groups than in the control groups. The histological changes observed in the 80-mg/kg and 120-mg/kg groups in this study were mild. Regarding the scoring of the histological changes, the 120-mg/kg group showed higher scores than the 80-mg/kg group, indicating that renal damage in the 120-mg/kg group was more severe than that in the 80-mg/kg group. Furthermore, the serum level of NGAL was higher in the 120-mg/kg group than in the 80-mg/kg group. Moreover, the most pronounced histopathological changes among all rats dosed with GM were observed in a rat of the 120-mg/kg group that showed the highest NGAL (363.7 ng/mL), Cr (3.84 mg/dL), and BUN (184 mg/dL) values. Based on these results, it appears that a 120-mg/kg dose likely causes more severe renal failure than the 80-mg/kg dose. The control groups showed extraordinary values that were clearly much lower than those of the GM groups, and were considered to be within the normal range. In the present study, the serum levels of α2M after the injection of turpentine oil were significantly increased in the 80-mg/kg group when compared to those in the control group; however, the concentrations did not differ significantly between the 120-mg/kg and control groups (Figure 2). Furthermore, the AUC of the 80-mg/kg group was significantly higher than that of the control group, but no significant difference was observed between the 120-mg/kg and control groups (Table 3).
It has been reported that following the intravenous injection of 125I-labeled α2M to rats, α2M accumulated more in inflammatory tissues than in normal tissues, and it appeared to accumulate in greater amounts in the kidney [23]. This is in accord with the results of the present study in which rats with induced severe renal injury accumulated more α2M in their kidneys than normal rats, and the serum α2M concentrations in rats of the 120-mg/kg group did not differ significantly from those in the control rats. Thus, the serum concentrations of α2M are not always elevated during renal failure, and appear not to be elevated in severe renal impairment. The t1/2 in the 80-mg/kg group was almost the same as that reported in a previous report [24]. However, the t1/2 in the 120-mg/kg group was longer than that in the 80-mg/kg group. The extended t1/2 of the 120-mg/kg group was likely due to the higher serum level of α2M in the final elimination phase compared to the 80-mg/kg group. Nonetheless, the t1/2 was not significantly different between the 80-mg/kg or 120-mg/kg groups and their respective control groups. A limitation of the present study is that the mechanism underlying why the elimination rate did not change remains unclear, and further investigations are needed to clarify this point.
Our findings suggest that a dose of 120-mg/kg group likely causes more severe renal failure than a dose of 80-mg/kg. The serum concentration of α2M does not necessarily increase in rats with renal failure, leading us to consider that the concentration may remain unchanged even in cases of severe acute renal failure.
Not applicable.


