Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Dirk Michael Forner* and Luisa Carlotta Mack
Received: August 29, 2023; Published: September 07, 2023
*Corresponding author: Dirk Michael Forner, Evangelisches Krankenhaus Kalk, Department of Gynaecology, gynaecologic Oncology and Obstetrics, Buchforststrasse 2, D-51103 Köln, Germany
DOI: 10.26717/BJSTR.2023.52.008296
Depending on the genesis and presence of HPV, vulvar carcinomas develop into two different morphological types. These are keratinising (kSCC; ICD 8071) and non-keratinising squamous cell carcinomas (nkSCC; ICD 8072). The present study uses cancer registry data from Germany over 10 years to investigate whether the morphological presentation by itself can predict prognosis. The study included a total of 13111 datasets from the years 2004 - 2014, 11807 (89.9%) had a kSCC and 1312 (10.0%) an nkSCC. Although G3 tumours were more common in nkSCC (33.8%) than in kSCC (17.6% P < 0.05) median overall survival was slightly worse for kSCC (101 months) than for nkSCC (109 months), and five year overall survival was 61 and 68%, respectively (log-rank p < 0.01). Morphologic typing of keratinizing versus non-keratinizing carcinomas allows the prediction of a better prognosis for the non-keratinizing malignancies. However, compared to molecular markers P16 and P3 and 50, the distinction appears less accurate. Nevertheless, this morphological classification can be of help when molecular markers are not available.
Abbreviations: SCC: Squamous Cell Carcinoma; VIN: Vulvar Intraepithelial Neoplasia; OS: Survival Were
Vulvar carcinoma is one of the rare genital malignancies, accounting for less than 5 % of them. Still, in Germany, more than 3 000 new cases have recently been observed per year, with the numbers practically doubling between 2003 and 2011. In the neighbouring countries, a similar rise has only been seen in the Netherlands, whereas the number of new cases has not increased in other countries [1]. In Germany, a particular increase was observed in the age group of 50-60 year old women, where the relative increase was twice as high as in all other age groups. Invasive squamous cell carcinoma (SCC) is usually preceded by precancerous vulvar intraepithelial neoplasia (VIN). Two types of VIN can be distinguished: Human papillomavirus (HPV)-induced intraepithelial lesions (uVIN/ HSIL) are the predominant type, accounting for about 80% of VIN [2-7]. The HPV-independent differentiated VIN (dVIN) is frequently associated with skin diseases such as lichen sclerosus [8-11]. The cumulative risk of developing invasive SCC within 10 years differs significantly, being 9.7 % for uVIN/HSIL, whereas for dVIN it amounts to 50% [12]). Furthermore, when progressing, HSIL develops into non-keratinising squamous cell carcinomas, whereas dVIN is associated with keratinising SCC. Within the context of modern histopathology, the distinction between the two types is based less on morphology than on the molecular markers p16 and p53. However, these are not always available. The present study will investigate whether the distinction between the two histo-morphological groups allows us to draw conclusions about prognosis and overall survival.
For this study, the data sets of the German Centre for Cancer Registries, Epidemiological Cancer Registries data with the main diagnosis vulvar carcinoma (C51 ICD-10) from 01.01.2004 - 31.12.2014 were collected and examined. All patients for whom survival data were available until at least 2017 were included. Data sets of patients were excluded if the tumour classification according to FIGO was incomplete or when the diagnosis or final data could not be collected. Patients were also excluded if the tumour morphology was not clearly identified as keratinising (ICD 8071) or non-keratinising squamous cell carcinoma (ICD 8072). For the included patients, the time of diagnosis and the end of observation or death as well as the data concerning the tumour according to the TNM and FIGO classification were recorded. Lymph node metastases were classified as N1 if the inguinofemoral lymph nodes were affected according to the UICC and FIGO; metastases in the pelvis and paraaortally were evaluated as distant metastases (M1). Data analysis was performed with the statistical package XL-Stat for Windows using the Mann-Whitney test for non-normally distributed data, the Fisher exact test. Survival time analysis was performed using Kaplan-Meier estimation and the logrank test, the significance level being 0.05.
A total of 13111 data sets from the years 2004 - 2014 were included in the analysis. 11807 patients (89.9 %) presented with a keratinising SCC, in 1312 (10.0 %) a non-keratinising squamous cell carcinoma was described. The median age at the time of disease was 73 years (SD 14.7 years), 48% of cases had FIGO stage I, whereas FIGO stages II - IV were present in 14.6%, 16.7% and 6.6%, respectively. The two groups did not differ in these parameters. According to the morphology, G3 differentiation was more frequent (33.8%) in non-keratinising squamous cell carcinoma than in keratinising SCC (17.6 %, P < 0.05). The median survival for keratinising SCC was 101 months, whereas for non-keratinising SCC it was 109 months, and the overall survival (OS) was 61 and 68 %, respectively (log-rank P < 0.001, see Figure 1). These differences in overall survival were similar for all FIGO stages. For example, the 5-year survival rate (5YSR) for FIGO stage I was 75 % for kSCC and 82 % for nkSCC. Corresponding rates for stages II were 55 and 61 %, for stage III 43 and 47 % and for stage IV 27 and 31 %, respectively.
Figure 1 Kaplan-Meier analysis results show that overall survival differs significantly for keratinising (ICD 8071) and non-keratinising SCC (ICD 8072) (log-rank test p<0.001).
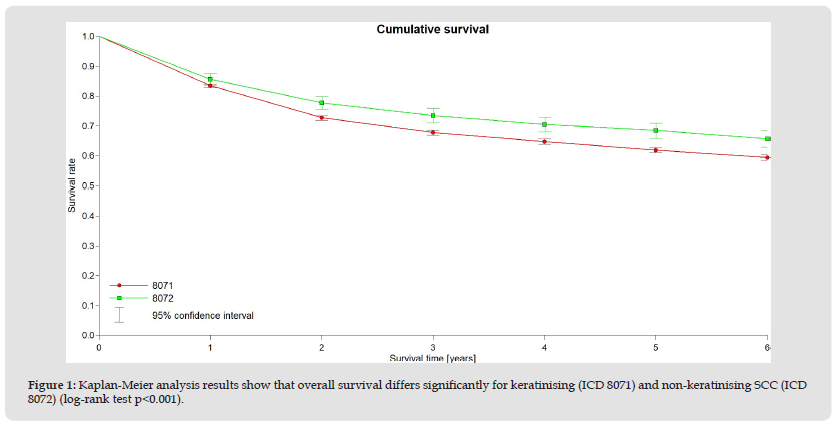
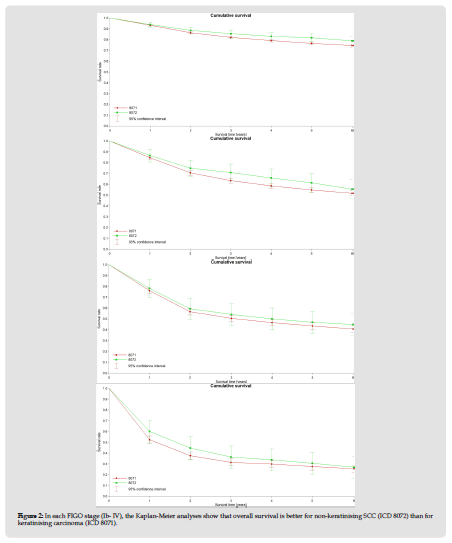
Vulvar cancer is a rare disease. Treatment is usually surgical or radiotherapy, but in both cases it is associated with considerable morbidity [13]. For these reasons, it is highly relevant for the gynaecological oncologists to identify factors that help to assess patients’ prognosis and risk of recurrence. In our work, we investigated to what extent morphological factors are suitable for differentiating patients and making statements about their prognosis. The 5-year survival of patients with non-keratinising SCC was found to be significantly better (68%) than that of patients with keratinising squamous cell carcinoma, although G3 tumours were less frequent in the latter group. Previously published studies have generally used molecular markers to describe the different types of vulvar cancer and investigated their clinical relevance (Figure 2). Jacek found that p16 overexpression but not the HPV status showed a correlation with prolonged overall survival, [14]. Further work also found that p16 expression as well as p53-apparition, were useful for differentiating risk groups, with p16 proving favourable and p53 negative [15,16]. Hence, the use of molecular markers allows a considerable separation of the groups. This was reflected in a difference in the 5-year OS of 50% versus 80 %. In addition, the study identified a third group in which negative HPV status and p53 wild-type showed a less pronounced difference from the HPV-positive group. This was expressed in hazard ratios of 2.1 and 2.43, respectively [16]. The results largely correspond to those of the AGO-CaRE-1 study; here, too, the differences were significantly greater than in the purely morphological distinction we made [15].
Figure 2 In each FIGO stage (Ib- IV), the Kaplan-Meier analyses show that overall survival is better for non-keratinising SCC (ICD 8072) than for keratinising carcinoma (ICD 8071).
This is also in line with the results from New Zealand, where the 3-year DFS was 95% and 73% [17]. Strikingly more patients can be assigned to p16-positive and HPV-assigned carcinomas by molecular classification (30.2 - 44 %) [15,17], than was the case for nkSCC in our study. In our evaluation, this was only 10 % of patients who had non-keratinising squamous cell carcinoma. Studies investigating overall survival and recurrence rates of vulvar carcinoma have their limitations. For example, the increase in less radical surgical approaches avoiding the vulvectomy was associated with a worse outcome in HVP-independent tumours, whereas this was not the case in HPV-dependent tumours [18]. Horne also showed that p16-positive patients had a better response to chemoradiation than p16-negative and p53-positive patients [19]. Whether these results can be transferred to morphological criteria is speculative. However, it shows that differentiating therapy is important. By analysing cancer registry data, it is possible to recruit extremely large collectives even for rare carcinomas such as vulvar carcinoma. With more than 13,000 data sets on overall survival, this is one of the largest collectives that have been studied for vulvar carcinoma. The greatest weakness is the quality of the data collected in the cancer registry and the large number of patients who could not be included in the analysis due to missing or incomplete data. In the German cancer registries, information on HPV, p16 and p53 is unavailable even though these molecular criteria would certainly allow a much more precise classification of patients with vulvar carcinoma into risk stratifying groups. The morphological determination of a keratinising or non-keratinising SCC is, however, generally available in every cancer patient whether or not molecular markers were analysed. This work shows, that a prognosis assessment is also possible on morphologic data alone, although the combination with the molecular markers would improve the statement.


