Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Fahim A Shaltout1*, Shimaa N Edris1, Mohamed E Nabil2 and Soha T Taha3
Received: August 23, 2023; Published: September 04, 2023
*Corresponding author: Fahim A Shaltout, Food Hygiene and Control Dept, Faculty of Vet. Med., Benha Univ, Egypt
DOI: 10.26717/BJSTR.2023.52.008286
Chicken meat is a popular, highly nutritious, and easily digestible source of protein. Chicken meat is a desirable target for direct or indirect bacterial contamination at each stage of production, from rearing to ready-to-eat meal. Therefore, the present study was conducted to evaluate the bacteriological quality of 120 random samples of raw, chilled chicken cuts (breast, thigh, drumstick, and wings,30 of each) sold in Benha city’s local markets and their risk to public health. The obtained results indicated that the examined chicken cuts meat samples exhibited the lowest safety with the highest bacterial counts; where aerobic plate count (APC), coliform count (CC), S. aureus and C. perfringens counts (CFU/g) were 1.9×104, 18x102, 7.2x102 and 1.1x103 for breast samples; 63x104, 22x102, 9.1x102 and 1.8x103 for drumstick samples; 85x104, 28x102, 12x102 an 2.8x103 for thigh samples; 8.6x104, 20x102, 10x102 and 1.5x103 for wing samples, respectively. The thigh samples also had a significantly higher rate of E. coli and salmonella than the other chicken samples (50 and 10 %, respectively). In addition, eight of the isolated S. aureus strains demonstrated an affinity for producing enterotoxins that were typed as SEA, SEC, and SED with a prevalence of 62.5%, 12.5%, and 25%, respectively. Samples were evaluated in accordance with Egyptian standards and their suitability for human consumption was documented. Therefore, strict hygienic measures should be implemented to reduce the affinity and dangers posed by bacteria that cause food poisoning.
Keywords: Food Safety; Poultry Meat; Egypt
Abbreviations: APC: Aerobic Plate Count; CC: Coliform Count; GIT: Gastrointestinal Tract; STEC: Shiga Toxin- Producing E. coli; HUS: Hemolytic Uremic Syndrome; RV broth: Rappaport Vassilidis Broth; TBX Agar: Tryptone Bile X-Glucoronide Agar; TSC Agar: Tryptose Sulfite Cycloserine Agar; LS: Lactose Sulfite
Chicken accounts for approximately two thirds of the world’s total production of animal protein, which helps alleviate the problem of lack of animal meat (Ruban, et al. [1,2]). The widespread consumption of poultry meat can be attributed to its high quality, easily digestible proteins, which include essential amino acids; its low fat and cholesterol content; and its considerable content of minerals and vitamins (Hassan, et al. [3,4]). Poultry meat is considered perishable because it contains animal proteins that are easily degraded, a favorable PH, and physicochemical characteristics that promote the growth of microorganisms (Odeyemi, et al. [5]) Furthermore, poultry meat has been easily contaminated during evisceration from gut bacteria as salmonella and/or personal cross contamination, or by the surrounding environment from air or water bacteria increasing the incidence of foodborne microorganisms such as Salmonella, S. aureus, E. coli, and C. perfringens which remain a public health issue with zoonotic importance (Kim, et al. [6,7]). Among most prevalent bacteria contaminants poultry meat products, contamination with Enterobacteriaceae, which includes E.coli and salmonella and is a common resident of the gastrointestinal tract (GIT) of chicken, occurs not only during slaughtering but also in wet markets (Tum [8]).
Salmonella and E. coli infections are typically accompanied by clinical symptoms of gastroenteritis, including vomiting, abdominal pain nausea, headache, and fever (Adeyanju, et al. [9]). In addition, Shiga toxin- producing E. coli (STEC) can cause advanced persistent diarrhea as well as hemolytic uremic syndrome (HUS) (Shah, et al. [10]). Additionally, gram-positive bacteria, particularly Staphylococcus aureus and C. perfringens, are one of the main contaminants of meat and meat products. One of the most common types of bacteria found on people’s skin and in their environments (dust, water, air, feces, or on utensils) that can contaminate food is Staphylococcus aureus (Xu, et al. [11]). Staphylococcal enterotoxins encoded as SEA, SEB, SEC, SED, SEE, are primarily associated with S. aureus food poisoning and are responsible for emesis, nausea, diarrhea, and abdominal cramps for about 24-48h (Shijia, et al. [12]). On the other hand, Clostridium perfringens, which is typically present in the GIT of food animal, can contaminate meat and meat products through improper practices that occurred during slaughtering and evisceration and may be linked to fecal contamination (Ohtani, et al. [13]). It is classified as a pathogenic bacterium that causes food poisoning because a large number of vegetative cells can survive the acidic PH of the stomach and produce enterotoxin in the small intestine (Ameme, et al. [14]); causing acute diarrhea and severe abdominal pain 8-24 hours after ingestion of the contaminated meat products (Labbe, et al. [15]). As the result, the current study aimed to assess the bacteriological quality of chicken cuts (breast, thigh, drumsticks and wings) and their suitability for human consumption in relation to Egyptian standards.
Collection of Samples
A total of 120 random samples of different raw, chilled chicken meat cuts represented by breast, thigh, wing and drumstick (30 of each) were collected from different poultry butchers located in Benha city. Each sample was presented to the following steps for evaluation of their bacteriological quality.
Preparation of Samples (ISO 6887-1: 2017)
Tenth fold serial dilutions were prepared on sterile peptone water (0.1%); from which the following parameters were examined.
Aerobic Plate Count “APC” According to ISO 4833-1 (2013)
On APC agar and incubated at 30±1OC for 72h. The Aerobic Plate Count (APC) per gram was calculated on plates containing 15 – 300 colonies and each count was recorded separately.
Coliform Count “CC” According to ISO 4832, 2006
On Violet red bile agar and incubated at 37±1OC for 24h. Suspected colonies, which showed purplish - red colonies surrounded by a red zone of precipitated bile acid, were enumerated to obtain coliforms count /g.
Prevalence and Enumeration of Enteropathogenic Escherichia Coli
Was performed according to ISO 16649-2 (2001) included plating on Tryptone Bile X-glucoronide agar (TBX agar) followed by incubation at 44oC for 24h. Suspected colonies, which showed Greenish-blue colonies were enumerated to obtain coliforms count /g.
Detection of Salmonellae was Performed According to ISO 6579 (2017)
Prepared sample was incubated in buffered peptone water broth at 37°C ± 1°C for 18 ± 2 hours, then transferred to Rappaport Vassilidis broth (RV broth) and incubated at 43°C\ 24hr. One ml of enriched sample was plated on selective XLD agar and Brilliant Green agar, and incubated at 37°C\24h, plates were examined for suspected Salmonella colonies which then isolated for confirmation. Suspected purified salmonella colony was cultured on three biochemical media represented by (TSI agar, Urea agar, and L-Lysine decarboxylation medium) and incubated at 37°C\24hrs.
Enumeration of Staphylococcus Aureus
Was performed by plating 0.1 ml on Baird Parker agar. Suspected colonies were purified and subjected for further biochemical identification following ISO 6888- 1 [16].
Isolates by Detection of Enterotoxins Producing S. Aureus Reversed Passive latex Agglutination Kit (SET-RPLA) Test Was performed on 24 purified S. aureus isolates according to (Igarashi, et al. [17]).
Detection and Enumeration of Viable C. Perfringens
Was performed by inoculating one ml of the previously prepared serial dilution on Tryptose sulfite cycloserine agar (TSC agar), followed by anaerobic incubation at 37OC for 20-22h. Suspected colonies were purified and subjected for identification on Lactose sulfite (LS) broth inoculation, which appeared as black ppt and gas formation according to ISO 7937 [18].
Statistical Analysis
The obtained data was statistically treated by one-way ANOVA using SPSS software for Windows (Version 16). Duncan’s post hoc analysis was used to analyze the data, with a p-value of 0.05 being regarded statistically significant (Steel, et al. [19]).
(Table 1) showed that the APC, coliform count and C. perfringens count (CFU/g) was significantly (P ≤ 0.05) higher in the thigh samples than in the drumstick, wing, and breast samples, in that order. In terms of S. aureus count (CFU/g), there was no statistically significant difference (P > 0.05) between the thigh and wing samples, but there was (P ≤ 0.05) between the drumstick and breast samples. According to EOS, 2019, the breast samples had acceptable microbiological quality (66.6%, 76.6%, 90%, and 90% for APC, CC, Staphylococcus aureus, and Clostridium perfringens counts, respectively) when compared to the other chicken meat cuts (Table 2). (Figure 1) depicts that thigh samples had the highest incidence (50%) of isolated E. coli, while breast samples had the lowest incidence (33 %). While, while a high rate of salmonella was found in 3 samples of thigh (10%) but failed to be detected in breast samples. In addition, (Table 3) shows that out of the 24 isolated S. aureus strains, 8 (33.3%) showed positive affinity to produce enterotoxins, with 5 (62.5%) being positive for SEA, 1 (12.5%) being positive for SEC, and 2 (25%) being positive for SED. (Table 1). Aerobic plate counts, Coliform, Staphylococcus aureus and Clostridium perfringens counts for chilled chicken meat cuts in Benha city (Table 4).
Table 1: Aerobic plate counts, Coliform, Staphylococcus aureus and Clostridium perfringens counts for chilled chicken meat cuts in Benha city.
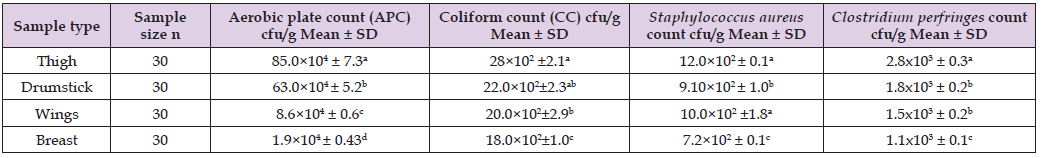
Note: (a, b, c) Small different litters mean significant difference of chicken meat cut samples (P≤0.05).
Table 2: Samples of chilled chicken meat cuts categorized based on EOS, 1651/ 2019 microbiological guidelines.
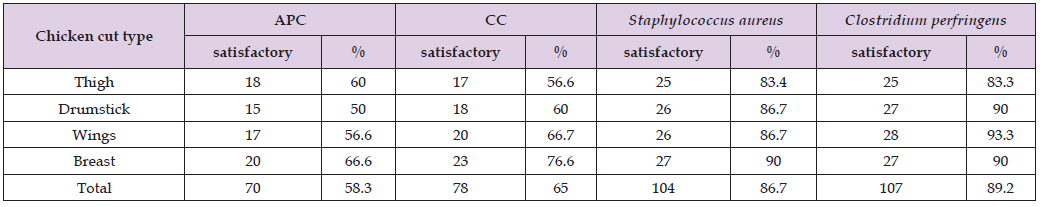
Note: Key to classification (EOS,2019).
Note:
1. n.of salm: number of isolated salmonella,
2. % of salm: percentage of isolated salmonella,
3. n. of E.coli: number of isolated E.coli,
4. % of E.coli: percentage of isolated E.coli.
Note:
1. APC: Aerobic Plate Count.
2. CC: Coliform count.
3. EOS: Egyptian Organization for Standardization and Quality Control.
Chicken meat may be loaded with different foodborne bacteria through all of the processing point’s starts with slaughtering and ending to the cooking and serving steps (Lianou, et al. [20]); therefore, continuous microbiological assessment of the retailed poultry meats is recommended. Referring to the recorded results of APC (CFU/g), nearly similar results were reported by (Hassanin, et al. [21]) (6.13x104 CFU/g in breast samples, while it is considered lower than the current obtained results for drumstick and thigh (7.47x104, 6.51x104); (Shaltout, et al. [22]) (5.9x105 and 7.1x105 in breast and thigh samples, respectively). While higher results were recorded by (Wahbah [23]) (5.5x106 and 6.8x106 for breast and thigh samples, respectively), and (Hassanin, et al. [24]) (8.16x105, 7.85x105, 6.76x105 and 5.58x105 in wings, drumsticks, thigh and breast samples, respectively). On the other hand, lower counts were reported by (Atia [25]) (9.28x103 and 2.91x104 in breast and thigh samples, respectively), and (Hosny, et al. [26]) (2x104 and 6x103 in drumstick and wing samples, respectively).
Detection of coliform bacteria in meat products usually indicating the environmental sanitation level around food processing area, or become as a sign of water pollution, personal hygiene and cross contamination may be (Feng, et al. [27]). Referring to the currently obtained results of coliform count (CFU/g), they were in line with the recorded results by (Shaltout, et al. [28]) (37.3x102 (wing), 21.6x102 (breast) and 27.7x102 (thigh)), and (Hassanin, et al. [24]) (2.66×103, 2.12×103, 2.01×103 and 1.84×103 for wing, drumstick, thigh and breast, respectively); while, they were higher than those recorded by (3x102 (wing) and 1x102 (drumsticks)). Contamination of chicken carcass with E. coli indicates unhygienic environment and possible fecal contamination during slaughtering, manual evisceration, and handling as this bacteria is naturally inhabitant in warm blooded animal gut and in intestine of human (Whyte, et al. [29]). The current prevalence is higher than those recorded by (Hassanin, et al. [24]) (8% (breast), 8% (thigh), 16% (wing) and 18% in (drumsticks)), while lower than those recorded by (Afify [22]) (12% in breast and 18% in thigh samples).
Salmonella is the second most common foodborne pathogen associated with zoonotic enteric human infection, which can occur as a result of cross-contamination with internal organs during evisceration or contamination during scalding or deboning (Zishiri, et al. [30]).The current prevalence of Salmonella species in the examined samples is higher than those recorded by (Shaltout, et al. [31]) (8% of thigh samples), but higher prevalence was reported in the recorded results of (Atia [25]) (8% and 20% of breast and thigh samples, respectively), and (Elsisy [32]) (20 and 25% of breast and thigh samples, respectively). The presence of S. aureus in meat and meat products is indicative of poor hygienic practices, which are primarily the result of improper personal hygiene and a contaminated environment caused by knives, workers’ hands, or inadequately cleaned equipment (Perry, et al. [33]). The present results of Staphylococcus aureus count (CFU/g) are less than the recorded results of (Shaltout, et al. [28]) (2.5 x103 in thigh, 2.4x103 in breast and 2.17x103 in wing), but the current prevalence came higher than those of (Shaltout, et al. [34]) (10 and 4% of breast and thigh samples, respectively), and (Mohamed, et al. [35]) (4.11x103 and 2.53x103 for thigh and breast samples, respectively); while came in line with those recorded by (Afifi-Dina (2016) (34.3% of the examined chicken cut samples, where its S. aureus enterotoxigenicity classification by SET-RPLA test revealed detection of SEA, SEB and SEC, and (Hassanin, et al. [21]) (1.9×102, 2.2x102 and 2.6x102 for breast, thigh and drumsticks, respectively).
Clostridium perfringens (C. perfringens) is commonly found in soils, dust, foods (especially raw meat), human intestinal tracts (10%- 30% of adults), and domestic animals (40 percent -80 percent in poultry). Under adverse conditions, C. perfringens can produce spores that are highly resistant to environmental stresses. Infection is typically acquired at schools and camps, or from food caterers or restaurants where large quantities of food are prepared and kept warm for extended periods of time (Mokhtari, et al. [36]). Therefore, the presence of this bacterium is primarily regarded as fecal contamination. The present prevalence of C. perfringens was lower than the recorded results of (Zakaria [37]) (25% and 35% of breast and thigh, respectively), and (Nabil [38]) (40 and 52% of the examined breast and thigh, respectively); while was nearly similar to (Afshari, et al. [39]) who detected C. perfringens in 15.5% of the examined chicken meat samples. Moreover, lower results were recorded by (Thangamani, et al. [40]) who detected C. perfringens in 3.81% of the examined chicken meat samples. Variations in results among authors may be attributable to differences in sample origin, hygienic practices, personal hygiene, and sample processing status [41-47].
The results indicate that thighs had the highest levels of contamination, followed by drumsticks, wings, and breasts, in that order. This study indicates that fresh chicken meat cuts can harbor a variety of food-poisoning bacteria, resulting in substandard quality and public health risks.


