Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Georgios Komisopoulos1*, Panayiotis Mavroidis2, Vasileios Simopoulos3, Giorgos Kyrgias1, Nikos Papanikolaou4 and Kyriaki Theodorou1
Received: June 17, 2023; Published: August 17, 2023
*Corresponding author: Georgios Komisopoulos, Department of Medical Physics, Medical School, University of Thessaly, Larissa, Greece
DOI: 10.26717/BJSTR.2023.52.008225
Purpose: This study aims at comparing the treatment quality of breast conformal radiotherapy (CRT) with
sequential boost to tumor bed against intensity-modulated radiation therapy (IMRT) with simultaneously
integrated boost to tumor bed by analyzing their corresponding dosimetric and radiobiological metrics.
Subjects and Methods: This work includes 20 primary left-sided breast cancer patients (10 with superclavicular
node involvement (SCV) and 10 without SCV). In total 20 CRT plans and 20 IMRT plans were
created. In the CRT plans, the whole breast with or without SCV irradiation receives a dose of 50Gy in 25
fractions with a sequential boost to the tumour bed of 10Gy in 5 fractions. In the IMRT plans, the whole
breast receives 50Gy and the tumor bed 60Gy in 25 fractions. Each plan was evaluated in terms of PTV
coverage, primary tumour conformity index, maximum plan dose, ipsilateral lung dose (Dmean, V20Gy, V10Gy,
V5Gy), heart dose (Dmean, Dmax, V15Gy), contralateral lung dose (V5Gy) contralateral breast dose (max, D5%) and
spinal cord dose (D0.03cc). The tumor control and normal tissues complication probabilities (TCP and NTCP)
were calculated for each plan.
Results: IMRT created more conformal plans in all cases, (conformity index: CRT=0.35, IMRT=0.75
with SCV and CRT=0.33, IMRT=0.76 without SCV). CRT plans delivered higher heart V15Gy (CRT=6.5%,
IMRT=5.2% with SCV and CRT=1.6%, IMRT=0.7% without SCV). CRT plans delivered higher V20Gy ipsilateral
lung doses with SCV (CRT=15.0%, IMRT=12.8%). Also, IMRT plans delivered lower D0.3cc spinal cord doses
(CRT=17.3Gy, IMRT=7.1Gy with SCV and CRT=2.6Gy, IMRT=0.5Gy without SCV). The NTCP values of heart
and ipsilateral lung were lower for IMRT (0.03% and 1.87%) than CRT (1.10% and 4.70%) with SCV,
respectively.
Conclusions: IMRT plans showed superior dose conformity, lower NTCP values and shorter treatment
duration. Especially, when there is SCV node involvement, it should be considered as the treatment of
choice.
Keywords: Breast Cancer Radiotherapy; Intensity-Modulated Radiation Therapy; Normal Tissue Complication Probability; Treatment Plan Optimization
Abbreviations: CRT: Conformal Radiation Therapy; IMRT: Intensity-Modulated Radiation Therapy; PTV: Planning Target Volumes; OAR: Organs at Risk; DVHs: Dose Volume Histograms; TCP: Tumor Control Probability; NTCP: Normal Tissue Complication Probability
A significant number of breast cancer patients are still treated using 3D-Conformal techniques [1,2] with various approaches regarding treatment parameters such as: angles of the tangential fields, sequential or integrated boost to the tumor bed, fractionation scheme [3-6]. However, the last years the use of highly conformal treatment modalities such as MLC-based IMRT or Tomotherapy have gained popularity. Intensity modulated radiation therapy (IMRT) produces more conformal distributions as compared to conformal radiation therapy (CRT) techniques by reducing radiation dose and toxicity to nearby critical organs [7-11]. IMRT may therefore significantly improve dose delivery since it employs significantly more degrees of freedom in conforming the dose distribution to the demands imposed by the dose objectives and constraints used during treatment planning [2-14]. It has been demonstrated that intensity modulated radiotherapy (IMRT) can significantly reduce radiation dose and toxicity to critical organs producing better results than conventional and simple conformal radiation therapy techniques. Expecially, the sparing of heart has gained interest by the radiotherapy community [15-18]. In the literature, there are reports on the physical comparison of dose distributions delivered by advanced radiotherapy techniques including fixed-beam intensity-modulated radiotherapy (IMRT), non-coplanar volumetric modulated arc therapy (NC-VMAT), multiple arc VMAT (MA-VMAT), and tomotherapy (TOMO) [19-23]. Those studies indicate that still there is no technique that performs better than the other at all aspects. This finding is also supported by studies that incorporated tumor control and normal tissue complication probabilities (TCP and NTC) [24-27].
Furthermore, the expected clinical impact of breathing motion, patient setup uncertainties and risk for secondary malignancies were among the factors that have not been thoroughly studied [28,29]. Many radiotherapy centers are still debating whether to proceed to a change of their treatment methodologies being cautious about the cost-benefit issues. One of the purposes of this study is to indicate the margin of benefit in the expected clinical outcome for breast cancer cases, which stems from the much higher conformality of the MLCbased IMRT modality compared to the 3D-Conformal techniques. In this study the difference between a combination of a SIB and IMRT and the traditional conformal two-phase approach were compared, to determine if any technique provides an improvement in plan conformity. Furthermore, a translation of the observed differences to radiobiological terms will show the clinical impact of those differences on the treatment outcome.
In this study, the data of 20 primary left-sided breast cancer patients (10 with and 10 without supraclavicular radiotherapy (SCVRT)) treated at University General Hospital in Larissa were used. For the patients with SCV-RT the whole-breast – SCV volumes and the primary tumor volumes ranged from 407.6 to 2282.6 cm2 and 41.9 to 199.7 cm2, respectively. For the patients without SCV-RT, the wholebreast volumes and the primary tumor volumes ranged from 353 to 1429.9 cm2 and 18.9 to 135.8 cm2, respectively. The range of breast sizes and shapes is typical of those encountered clinically. Computed tomography (CT) images were obtained in 3mm slices using a scanner with 16 detector arrays (Toshiba aquilion 16), with patients in the supine position on a breast board with both arms above their heads. Scanning was performed with free breathing. The attending radiation oncologist contoured the clinical target volumes (CTV) and the planning target volumes (PTV) for the whole-breast, whole-breast-SCV and tumour bed, subtracting 3 mm off the build-up region from the skin surface of the breast. Furthermore, OARs delineated included heart, lungs, contralateral breast and spinal cord.
Conformal Radiotherapy Plans
For the whole-breast plans, an isocentric technique was used with conventional coplanar opposed fields (medial and lateral) with a leaf margin of 3 cm to the skin side. Also, a Field-in-Field technique was used in order to facilitate better control of dose homogeneity. For the irradiation of the tumour bed, two coplanar fields were used keeping the same isocentre and selecting gantry and collimator angles according to its size and location. The energies, the gantry and collimator angles, the beam weights and the wedges were manually optimized for both PTVs. Mainly 6MV beams were used, however 10MV and 15MV were also used when necessary. The collapsed cone convolution dose algorithm was used to calculate the dose. Additionally for the SCVRT, 2 opposed fields were mainly applied in the anterior-posterior direction (AP and PA) with gantry angles close to 350° and 170°, respectively and energies 6MV for the AP and 15MV for the PA. Field in Field technique was also used where necessary. The CRT plans were performed using a standard regimen where the whole breast receives a dose of 50 Gy in 25 fractions of 2 Gy and the tumour bed receives a dose of 60 Gy in 30 fractions of 2 Gy. A typical CRT beam setup is shown in Figures 1 & 2.
IMRT with SIB Plans
An MLC-based dynamic IMRT plan was created for each patient using 3 lateral fields with 5 degrees step, 3 medial fields with the same step and 1 field fixed to tumour bed selected at gantry angle determined by the location of the tumour bed. All the plans use 6 MV photon beams and were developed with maximum number of control points per beam of 200 and minimum segment width of 1 cm. Those IMRT plans were developed using a sequential integrated boost (SIB) scheme where the tumour bed receives a dose of 60 Gy in 25 fractions of 2.4Gy and the whole breast or the whole breast and SCV receives a dose of 50 Gy in 25 fractions of 2Gy. Objectives were created at the inverse planning stage to ensure that both the whole breast PTV or the whole breast and SCV and the tumour bed PTV received 50 Gy and 60 Gy over 25 fractions, respectively. The Monte Carlo dose algorithm was used to calculate dose. A typical IMRT beam set up is shown in Figures 1 & 2.
Endpoints
Treatment plans were prescribed as follows: V50Gy to the whole breast or the whole breast and SCV PTV and V60Gy to the tumour bed PTV should be at least 95%, respectively [30]. Dose to the surrounding tissue should be kept as low as possible. For the IMRT plans the prescription was done to the mean dose of PTVbed (60Gy mean dose of PTVbed). Measured data for plan comparison included:
• The coverage of PTVs and the overall maximum plan doses.
• The lung doses to the ipsilateral lung in the form of mean
dose, V20Gy, V10Gy and V30Gy.
• The mean and the maximum heart doses as well as V15Gy.
• The dose to the contralateral lung in the form of D5Gy.
• The dose to the spinal cord in the form of D0.03cc.
• The dose to the contralateral breast in the form of mean dose
and D5%.
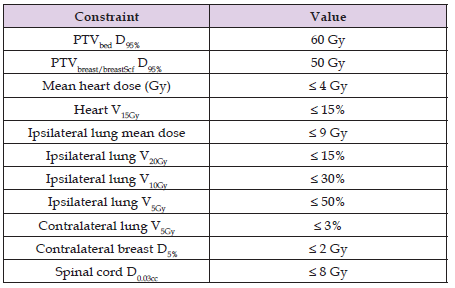
A summary of the prescribed doses and dose constraints to the organs at risk (OAR) is shown in Table 1. After creating the dose-volume histograms (DVHs) and using the required parameters, the Conformity index Lomax and Scheib for the tumour bed was calculated [30,31]. Conformity index = TVRI/VRI and it is defined as the ratio between the target volume covered by the reference isodose (TVRI) and the volume of the reference isodose (VRI). An index value of 1 indicates ideal conformity. In this study, the reference isodose used was the 57 Gy (95% of 60 Gy) isodose line. For two typical cases of patients without and with SCV involvement, the DVHs of the targets and primary organs at risk (OAR) are shown in Figure 3.
Table 1: Summary of the dose constraints and clinical goals for the different targets and organs at risk used in treatment plan optimization.
Statistical Method
All the simple statistical values (average, standard deviations, range) of the different dose metrics were taken from the Monaco Treatment Planning System (version 5.11.03, ELEKTA), using dose volume histogram information and volumetric data. The results were analyzed using a paired t-test. A resultant p-value of <0.05 implied a statistically significant difference.
Radiobiological Models
The dose-response relation of tumors and normal tissues is described by the Poisson model based on the following mathematical expression: [32]

where P(D) is the probability of response for a voxel in a tumor or OAR when it is irradiated uniformly with a dose D. In Eq. (1), D2Gy is the 2 Gy equivalent dose and it is calculated by the following equation: [32]

where D is the total voxel dose, d is the corresponding dose per fraction and α/β is a parameter that expresses the fractionation characteristics of that organ.
The probabilities of tumor control, PT and overall benefit, PB, are estimated by the following expressions: [33-36]

where Ntumors is the total number of tumors and M is the total number of voxels or subvolumes of the tumor.
The probabilities of normal tissue injury, POAR and overall injury PI, for a number of OARs is expressed by the following equation: [33-36]

where Norgans is the total number of OARs and M is the total number of voxels or subvolumes of the OAR. is the fractional subvolume of the organ being irradiated.
To express the radiobiological effectiveness of a given dose distribution in dosimetric terms, the value of biologically effective uniform
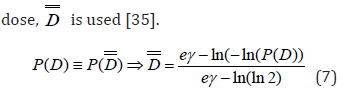
The overall quality of a treatment plan is evaluated by the complication- free tumor control probability (P+), which represents the probability of achieving tumor control without causing damage to normal tissues [8,9]. The P+ index can be calculated by the following equation: [36]
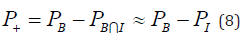
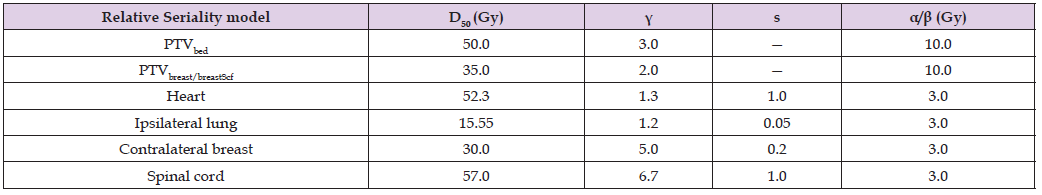
The dose-response parameters of those models are the D50, which is the dose for having 50% response and , which is the maximum normalized dose-response gradient. In the Relative Seriality model, the relative seriality parameter, s, which characterize the volume effect of the tissue is also involved. The dose-response parameters of the target and OARs that were used in this analysis are presented in Table 2. [37,38] The α/β was assumed to be 10 tumors and 3 for normal tissues.
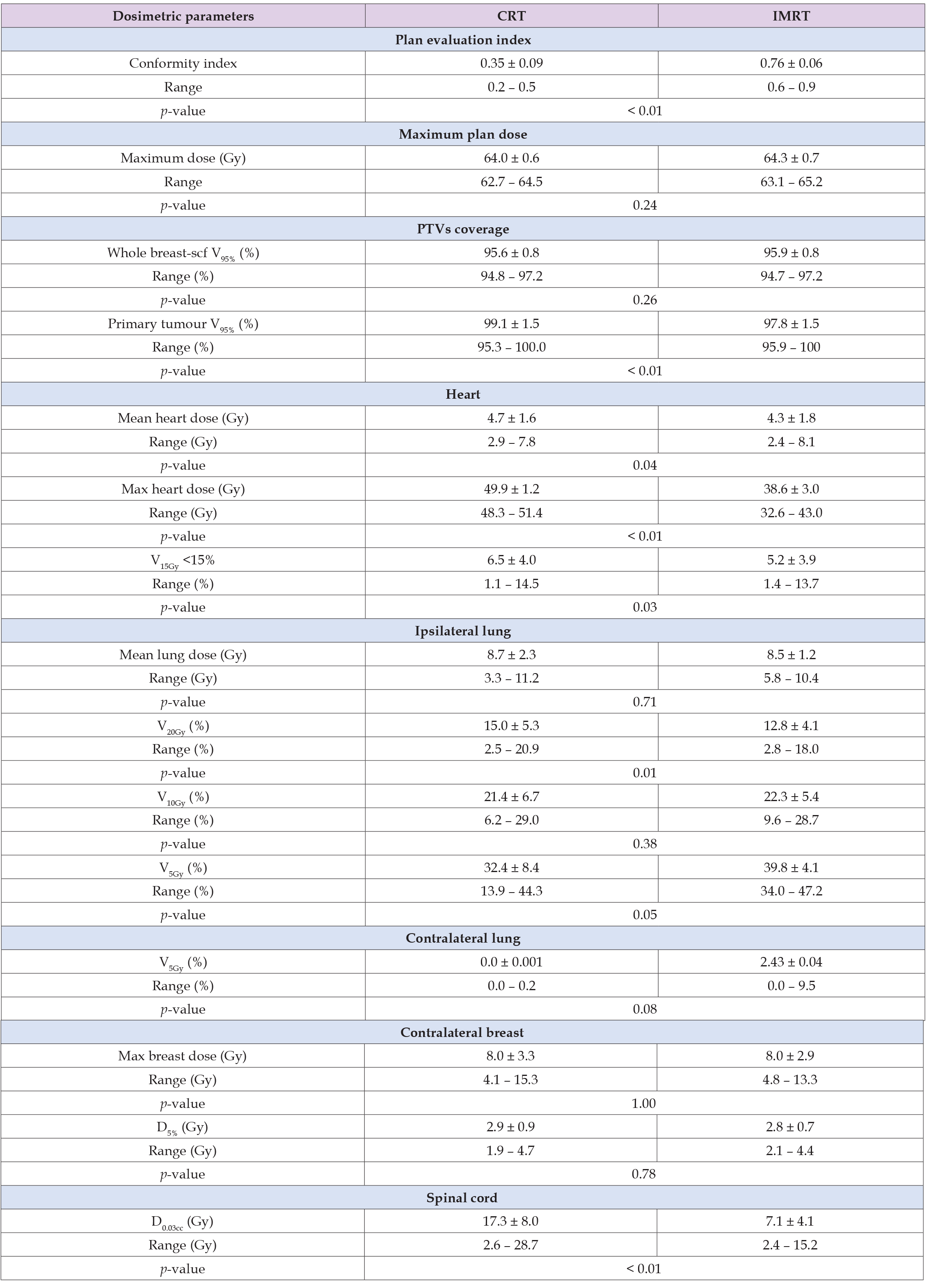
Table 3: Summary of the dosimetric and plan quality parameters for the two sets of plans without supraclavicular irradiation.
Patients without SCV Irradiation (Dosimetric Analysis)
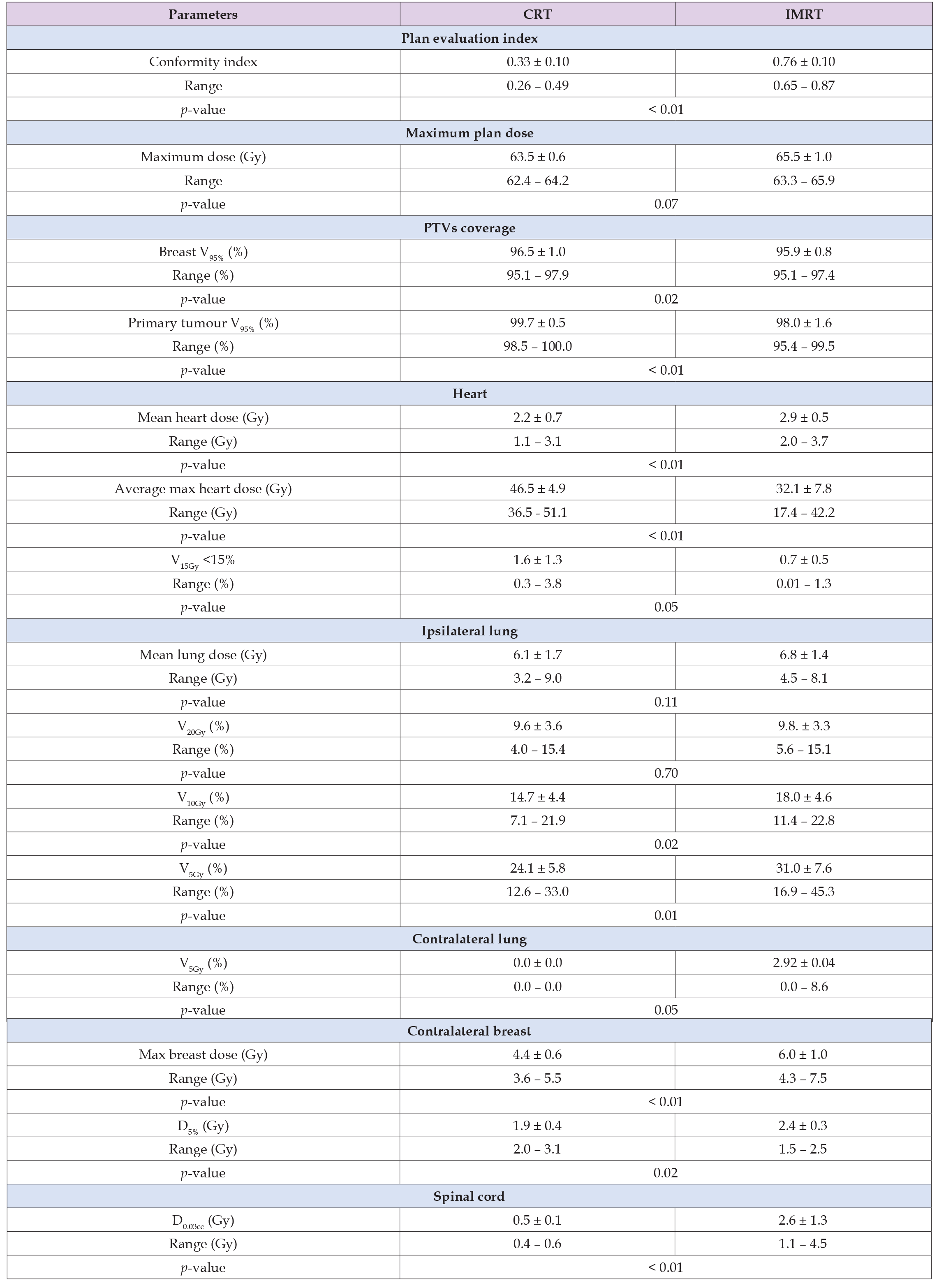
The breast PTV coverage of CRT plans is better in 8 of 10 cases (average CRT = 96.5%, IMRT = 95.9%) with statistically significant importance (p = 0.018) and the tumour bed coverage of CRT plans is better in 9 of 10 cases (average CRT = 99.7%, IMRT = 98.0 %) with statistically significant importance (p = 0.004) (Table 3). IMRT plans delivered higher maximum plan doses in 6 of 10 cases (average CRT = 63.5 Gy, IMRT = 65.5 Gy) without statistically significant importance (p>0.05) (Table 3). CRT plans delivered lower mean heart doses in 8 of 10 cases (average CRT = 2.2 Gy, IMRT = 2.9 Gy) with statistically significant importance (p = 0.009). On the contrary they delivered higher maximum doses in all cases (average CRT = 46.5 Gy, IMRT = 32.1 Gy) with statistically significant importance (p = 0.001). Also IMRT plans delivered lower V15Gy heart dose in 8 of 10 cases (average CRT = 1.6%, IMRT = 0.7% with statistically significant importance (p = 0.05) (Table 3).
IMRT plans delivered higher mean ipsilateral lung doses in 7 of 10 cases (average CRT = 6.1 Gy, IMRT = 6.8 Gy) and V20Gy in 6 of 10 cases (average CRT = 9.6%, IMRT = 9.8%) without statistically significant importance (p>0.05). IMRT plans also delivered higher V5Gy and V10Gy ipsilateral lung dose in 8 of 10 cases (average CRT = 14.7%, IMRT = 18% for V10Gy and average CRT = 24.1%, IMRT = 31.0% for V5Gy) with statistically significant importance (p = 0.016) and (p = 0.014) respectively. (Table 2). CRT plans delivered lower V5Gy in all cases (average CRT = 0.0 %, IMRT = 2.9 %) with statistically significant importance (p = 0.045) (Table 2). CRT plans delivered lower contralateral breast maximum doses in 9 of 10 cases (average CRT = 4.4 Gy, IMRT = 6.0 Gy). They also delivered lower D5% in 7 of 10 cases (average CRT = 1.9 Gy, IMRT = 2.4 Gy) with statistically significant importance (p = 0.001) and (p = 0.019) respectively (Table 2). CRT plans delivered lower spinal cord D0.3cc in all cases (average CRT = 0.5 Gy, IMRT = 3.3 Gy) with statistical significance (p = 0.001) (Table 2). In all cases, IMRT plans were considered more conformal when using conformity index. The average CRT plan conformity index was 0.33 compared with the average IMRT conformity index 0.76 with significance importance (p = 0.0001) (Table 3).
Patients With SCV Irradiation (Dosimetric Analysis)
The whole breast PTV coverage of IMRT plans is better in 8 of 10 cases (average CRT = 95.6%, IMRT = 95.9%) without statistically significant importance (p>0.05), whereas the tumour bed coverage of CRT plans is better in 8 of 10 cases (average CRT = 99.1%, IMRT = 97.8 %) with statistically significant importance (p = 0.007) (Table 4). IMRT plans delivered higher maximum plan doses in 7 of 10 cases (average CRT = 64.0 Gy, IMRT = 64.3 Gy) without statistically significant importance (p>0.05) (Table 4). IMRT plans delivered lower mean heart doses in 8 of 10 cases (average CRT = 4.7 Gy, IMRT = 4.3 Gy) with statistically significant importance (p = 0.042). They also delivered lower maximum doses in all cases (average CRT = 49.9 Gy, IMRT = 38.6 Gy) with statistically significant importance (p = 0.001). Also IMRT plans delivered lower V15Gy heart dose in 9 of 10 cases (average CRT = 5.2%, IMRT = 6.5% with statistically significant importance (p = 0.034) (Table 4). CRT plans delivered higher mean ipsilateral lung doses in 8 of 10 cases (average CRT = 8.7 Gy, IMRT = 8.5 Gy) without statistically significant importance (p>0.05) and V20Gy in 8 of 10 cases (average CRT = 15.0%, IMRT = 12.8%) with statistically significant importance (p = 0.012). On the contrary, CRT plans delivered lower V10Gy ipsilateral lung dose in 5 of 10 cases (average CRT = 21.4%, IMRT = 22.3%) without statistically significant importance (p>0.05) and V5Gy ipsilateral lung dose in 8 of 10 cases (average CRT = 32.4%, IMRT = 39.8%) with statistically significant importance (p = 0.05) (Table 4). CRT plans delivered lower V5Gy to the contralateral lung in all cases (average CRT = 0.0 %, IMRT = 2.4 %) without statistically significant importance (p>0.05) (Table 4). CRT plans delivered the same contralateral breast maximum doses (average CRT = 8.0 Gy, IMRT = 8.0 Gy) and the same D5%. CRT plans delivered lower spinal cord D0.3cc in all cases (average CRT = 17.3Gy, IMRT = 7.1Gy) with statistical significance (p = 0.003) (Table 4). In all cases, IMRT plans were more conformal. The average CRT plan conformity index was 0.35 compared with the average IMRT conformity index 0.76 with significance importance (p = 0.000) (Table 4).
Table 4: Summary of the dosimetric and plan quality parameters for the two sets of plans with supraclavicular irradiation.
Patients Without SCV Irradiation (Radiobiological Analysis)
The TCP of the tumor bed (PTVbed) was higher for the CRT plans in 6 of 10 cases (average CRT = 92.3%, IMRT = 91.1%) with statistically significant importance (p = 0.02), whereas the TCP for the breast PTV was on average better for the IMRT plans (average CRT = 94.6%, IMRT = 95.3 %) without statistically significant importance p = 0.48) (Table 5). A statistically important difference (p = 0.04) between the two modalities was observed in the NTCP of heart (CRT = 0.2% vs. IMRT = 0.0%). For ipsilateral lung, the NTCP values of the IMRT plans were lower than those of CRT (0.4 vs. 0.6%) but with no statistical significance (p = 0.65). For spinal cord and contralateral lung, the NTCP values were zero for all the plans. Examining the effectiveness of the plans as a whole, it is shown that the values of P+ were better for the IMRT plans (94.5 vs. 93.6%) but without statistically significant importance (p = 0.31). Similar were the findings for the values of PB (IMRT = 94.9%, CRT = 94.3%, p = 0.55). The corresponding PI values were also lower for IMRT (0.4%) than CRT (0.8%) with p = 0.24. Although the average values give the edge to IMRT, in most cases the differences were very small and in both directions.
Table 5: Summary of the radiobiological evaluation for the breast without supraclavicular irradiation for the CRT and IMRT radiation modalities.
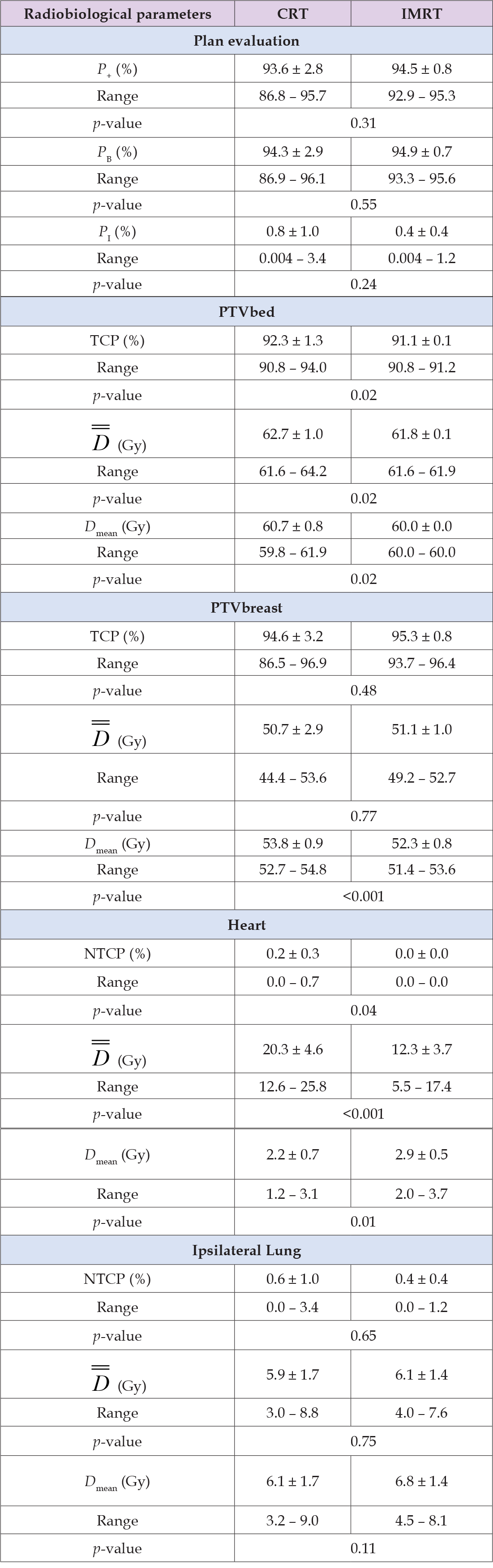
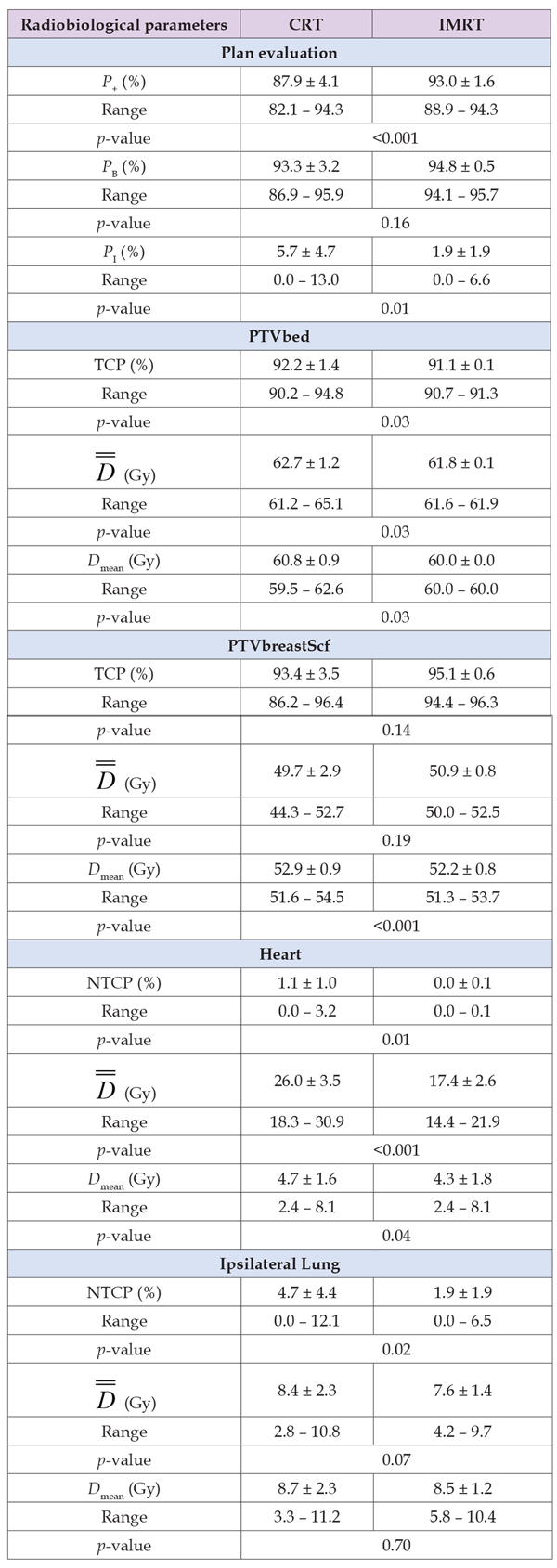
Table 6: Summary of the radiobiological evaluation for the breast with supraclavicular irradiation for the CRT and IMRT radiation modalities.
Patients With SCV Irradiation (Radiobiological Analysis)
The TCP of the tumor bed (PTVbed) was higher for the CRT plans in 7 of 10 cases (average CRT = 92.2%, IMRT = 91.1%) with statistically significant importance (p = 0.03), whereas the TCP for the breast PTV was on average better for the IMRT plans (average CRT = 93.4%, IMRT = 95.1 %) without statistically significant importance (p = 0.14) (Table 6). A statistically important difference (p = 0.01) between the two modalities was observed in the NTCP of heart (CRT = 1.1 vs. IMRT = 0.0%). For ipsilateral lung, the NTCP values of the IMRT plans were lower than those of CRT (1.9 vs. 4.7%) with statistical significance (p = 0.02). For spinal cord and contralateral lung, the NTCP values were zero for all the plans. Examining the effectiveness of the plans as a whole, it is shown that the values of P+ were considerably better for the IMRT plans (93.0 vs. 87.9%) with statistically significant importance (p < 0.001). The values of PB were better for IMRT (94.8%) than CRT (93.3%) but there difference was not statistically significant (p =0.16). However, a large difference was observed in the sparing of the primary organs at risk, where the corresponding PI values were much lower for IMRT (1.9%) than CRT (5.7%) with statistical significance (p = 0.01) (Table 6).
Table 2: Summary of the model parameter values for the breast cancer case. D50 is the 50% response dose, γ is the maximum normalized value of the dose-response gradient and s is the relative seriality, which characterizes the volume dependence of the organ.
The results of this study indicate that the dose coverage as expressed by V95% in the whole breast and tumour bed with and without SCV irradiation are very similar between the two sets of CRT and IMRT plans. However, the IMRT SIB plans demonstrate a significantly higher dose homogeneity (p < 0.001). For the cases including SCV irradiation, ipsilateral lung sparing was better for the IMRT plans regarding mean lung dose and V20Gy, which are the clinically relevant dose constraints but the differences were statistically significant only for the V20Gy. For the cases not including SCV irradiation, the mean lung doses and the V20Gy are quite similar. The CRT plans give better V10Gy and V5Gy with statistically significant importance but the differences in absolute values are so small that are not expected to have a significant impact in clinical outcome. In most cases, the IMRT plans give better results for the heart. When SCV irradiation is included mean heart dose, maximum heart dose and V15Gy are lower for the IMRT plans with statistically significant importance. Only the mean heart dose is lower for the CRT plans when SCV irradiation is not included but the differences between the two sets are very small. Those findings are in-line with other reports, which indicate a better sparing of heart by IMRT plans. [9,13,39] More specifically, Jöst et al showed that compared with the established 3D-CRT technique, a technique combining IMRT and VMAT allows for a decrease in dose, particularly of the mean heart and lung dose with comparable target volume acquisition and without disadvantageous low-dose load of contralateral structures.
Also, the IMRT plans give better results for the spinal cord in the cases with SCV irradiation. Although, the IMRT SIB plans deliver on average higher doses to the rest of the OARs (namely: contralateral lung and contralateral breast), those doses are at a level of very low clinical significance.
A significant practical implication is that in the IMRT SIB technique the overall treatment duration is reduced by 5 fractions. In busy departments that treat large numbers of breast cancer patients this can be important. The implementation of the IMRT technique does not require extra work on the behalf of radiation oncologists, however the IMRT plans require additional quality assurance testing by physics. For CRT plans, the size and location of both whole breast and tumour bed, influenced the choice of photons energy, however, for IMRT boost fields, 6-MV photons were used exclusively to treat the PTVs. The ability to create a comparable plan using only 6-MV photons, can be advantageous to departments in which higher energies like 15 MV are not available on every linear accelerator. There are very few studies analyzing the comparison between different treatment techniques in breast radiotherapy using radiobiological metrics. [7,32,37] The application of radiobiological measures on the two sets of 3D-Conformal and MLC-based IMRT treatment plans indicate that the TCP values of PTVbreast and PTVbed are very similar both in the cases with and without SCV irradiation. Regarding the primary organs at risk (heart and ipsilateral lung), the NTCP values were found to be similar in the cases without SCV irradiation but when SCV irradiation was needed the superiority of IMRT was very clear and statistically significant in terms of clinical outcome.
We have to acknowledge that a number of factors that were not examined by the present analysis such as the impact of use of deep-inspiration breath-hold (DIBH). More specifically, in a recent study that compared cardiac doses of different whole-breast optimization schemes including free-breathing (FB) tangential radiotherapy (TRT), (DIBH) TRT, and FB helical tomotherapy (HT), it was concluded that DIBH offers clear mean heart dose reductions (3.1Gy vs. 1.1Gy). [15] Another study examining the plans of 63 patients found a significant reduction of mean cardiac dose from 6.1Gy to 3.2Gy (p < 0.001) when DIBH was compared with FB. [16] Darapu and colleagues showed in their study that the difference in ipsilateral lung doses between FB and DIBH showed statistically significant p values, and the differences were found to be 7, 15.7, 11.8, and 10.7% for V5, V20, V30, and Dmean, respectively. [17] However, although the use of DIBH seems to have a beneficial impact on the quality of the treatment, another study showed that there is no significant difference in mean heart dose between VMAT-DIBH and TRT(field-in-field)-DIBH. [18] For patients who are not compliant with breath-hold the use of a 4D robust optimization has shown to limit the doses to cardiac and substructures during free-breathing RT with both IMRT/VMAT even if it cannot fully outperform DIBH or conventional 4D-CT-based planning with IMRT/VMAT in heart sparing. [40]
Based on this analysis, it is found that the MLC-based IMRT deliver better plans in terms of dose homogeneity than the 3D-Conformal modality. Plan quality is shown to be very similar in the case without SCV irradiation both in regards to the coverage of the PTVs and sparing of the organs at risk. However, when SCV irradiation was involved, IMRT could spare the organs at risk at a much higher level, which could be seen in both the dosimetric as well as in the radiobiological metrics.
There are no conflicts of interest.
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
All data generated or analyzed during this study are included in this published article.


