Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Wong Mei Wei1, Nabila Perveen1, Syed Atif Raza2 and Naeem Hasan Khan1*
Received: July 27, 2023; Published: August 10, 2023
*Corresponding author: Naeem Hasan Khan, Faculty of Pharmacy, AIMST University, Kedah D.A., Malaysia
DOI: 10.26717/BJSTR.2023.52.008206
Evaluate the percent purity of different brands of perindopril tablets available in the local market using UV
spectrophotometric and FTIR methods.
Objectives: To perform the quantitative determination of different brands of perindopril tablets available
in Malaysia by using UV spectrophotometric and Fourier transform infrared spectroscopy methods and to
compare the quality of perindopril tablets available in Malaysia within the brands as well as with the British
Pharmacopeia limit. Also, to describe the structural characterization by infrared spectrum of absorption,
emission, and photoconductivity of perindopril tablets.
Methodology: Each brand of perindopril was weighed, and the average weight was noted down. Each
perindopril tablet was as per the protocol of U.S.P. and B.P. methods. The percentage deviation of individual
weight from the average weight was determined. The disintegration test was carried out that consisted
of a rack holding 6 glass tubes, each having a 10-mesh screen at its bottom. The tubes were raised and
lowered at a fixed rate in a water bath maintained at 37 ± 2°C. Six tablets were placed, one in each tube,
along with a plastic disk over each tablet. The perindopril sample was dissolved in methanol to produce
25 ml (800μg/ml). A volume of 1ml, 2ml, 4ml and 6ml of stock solutions of each brand of perindopril
was pipetted into 100ml volumetric flasks and the volume was made up with distilled water to obtain
working standard solutions in the concentration of 8μg/ml, 16μg/ml, 32μg/ml, and 48μg/ml. At the
wavelength of absorbance at 213 nm, the absorbance of the dilutions and standard preparation in a 1cm
cell was measured using a U.V. spectrophotometer. The percentage purity of the perindopril tablets was
calculated. The perindopril sample was dissolved in a small amount of methanol and added sufficient
methanol to produce 25 ml (800μg/ml). A volume of 1ml, 2ml, 4ml and 6ml of stock solutions of each
brand of perindopril was pipetted into 100ml volumetric flasks and the volume was made up with distilled
water to obtain working standard solutions in the concentration of 8μg/ml, 16μg/ml, 3μg/ml, and 48μg/
ml. The wavelength of absorbance at 213 nm, the absorbance of the dilutions and standard preparation in
a 1cm cell was measured using a spectrophotometer. The percentage purity of the perindopril tablets was
calculated. Structural characterization of perindopril tablets was done through FTIR.
Results: For the UV spectrophotometric method, Brand B, C, D, and E was within the limit specified by BP.
For the FTIR method, all the brands of perindopril obtained were found to have structural characteristics
similarity of perindopril. Conclusion: It was concluded that Brand B and Brand D were the good ones. Both
UV spectrophotometric and FTIR methods can be used for the assay of perindopril in tablets.
Perindopril is a non-sulfhydryl prodrug that belongs to the angiotensin- converting enzyme (ACE) inhibitor class of medications. Its systematic (IUPAC) name is (2S,3aS,7aS)-1-[(2S)2-[(2S)-1-ethoxy- 1-oxopentan-2-yl]amino]propanoyl]-2,3,3a,4,5,6,7,7a-octahydroindole- 2carboxylic acid [1]. The active ingredient, perindopril, occurs in a variety of salt forms, including perindopril erbumine, perindopril arginine, and perindopril tert-butylamine. However, all of these salts function in the same way. Among a class of drugs that began with the blockbuster captopril, perindopril has the added feature of being a smaller molecule than ramipril and trandolapril. Due to these properties, perindopril has a high affinity for the N- and C-terminal active sites of tissue and circulating ACE. It is possible to produce stability and shield patients from the serious cardiovascular events of myocardial infarction and stroke by penetrating the margin of advanced atheromatous plaques [2]. The chemical structure of perindopril is shown in Figure 1(a,b). The active metabolite of perindopril, the perindoprilat, which has a high affinity for ACE, is produced in the liver by the hydrolysis of perindopril. Perindopril has a lipophilic perhydroindole group that gives it excellent ACE-inhibiting properties [3,4]. It is available as tablets or liquid formulations. Perindopril belongs to a class of drugs known as ACE inhibitors. To facilitate more effortless blood flow, it acts by relaxing blood vessels. Perindopril is rapidly metabolized in the liver to perindoprilat (2S,3aS,7aS)-1-[(2S)-2-[(2S)1- hydroxy1-oxopentan-2-yl]amino]propanoyl]-2,3,3a,4,5,6,7,7a-octahydroindole- 2carboxylic acid) its active metabolite [5]. Due to extensive metabolization, only 4-12% of the dosage is recovered in the urine after oral administration. Perindoprilat, perindopril glucuronide, perindoprilat glucuronide, a perindopril lactam and two perindoprilat lactams are the six metabolites that have been found. Pharmacological activity only exists as perindoprilat. The two principal circulatory metabolites are perindoprilat and perindoprilat glucuronide.
Perindopril is a prodrug ester hydrolyzed to create the active metabolite perindoprilat. Perindoprilat was created to allow oral delivery because it is poorly absorbed from the gastrointestinal tract. After oral administration, perindopril has an absolute bioavailability that ranges from about 66% to 95%. A single oral dose of perindopril is rapidly absorbed, reaching peak plasma concentrations approximately an hour later and then swiftly excreted from the body in about six hours [6]. Three to four hours after delivery, perindoprilat achieves its peak plasma concentrations. Perindoprilat is extensively metabolized to other inactive metabolites, leaving just 17 to 20% of the oral dose as active medication. Perindoprilat is broadly circulating because most tissues effectively block ACE. A radiolabelled dose of perindopril is recovered in the urine and faeces in amounts of around 75% and 25%, respectively [6]. The latter could be unabsorbed perindopril or biliary excretion. Perindopril, perindoprilat, and other metabolites, some of which are glucuronide conjugates, account for renal excretion. Perindopril and perindoprilat have mean total body clearances of 31 and 41 to 46 L/h, respectively, whereas the mean renal clearance ranges from 3.0 to 3.7 L/h and 6.1 to 10.3 L/h [6]. Perindoprilat has a biphasic elimination pattern with mean distribution (ti/2a) and elimination (t½β) phase half-lives of 5 and 25 to 30 hours, respectively. The mean elimination half-life of perindopril is around 1.5 to 3 hours. The latter half-life most likely illustrates the potent ACE binding of perindoprilat. Concomitant use with it may cause bilateral or unilateral renal stenosis, genetic or idiopathic angioedema, extracorporeal treatments causing blood vessels to contract with negatively charged surfaces, history of angioedema associated with prior ACE inhibitor use [7].
a. Perindopril-Impurity A Synonyms: [2S-(2α,3aβ,7aβ)]-Octahydro-
1H-indole-2-carboxylic Acid; (2S,3aS,7aS)-2Carboxyoctahydroindole;
(2S,3aS,7aS)-Perhydroindole-2-carboxylic Acid;
Perindopril related compound A.
b. Perindopril-Impurity C Synonyms:(2S)-2-[(3S,5aS,9aS,10
aS)-3-Methyl-1,4-dioxodecahydropyrazino[1,2-a]indol2(1H)-yl]
pentanoic Acid;10aS-PerindoprilatDiketopiperazine;(S)-2-[(3S,5
aS,9aS,10aS)-3Methyl-1,4-dioxodecahydropyrazino[1,2-a]indol-
2(1H)-yl]pentanoic Acid, Perindopril Related Compound C (USP).
c. Perindopril-Impurity E Synonyms: (2S,3aS,7aS)-1-[(2S)-
2-[[(1S)-1-[(1Methylethoxy)carbonyl]butyl]amino]propanoyl]
octahydro-1H-indole-2-carboxylic Acid; Perindopril Isopropyl
Analog.
d. Perindopril - Impurity J Synonyms:
(2S,3aS,7aS)-1-[(2S)-2-Aminopropanoyl] octahydro-1H-indole-2
- carboxylic Acid.
Method for the Assay of Perindopril
There are few methods to assay the perindopril, which include Fourier-transform infrared spectroscopy (FTIR), UV Spectrophotometer, Thin Layer Chromatography (TLC), and High-Performance Liquid Chromatography (HPLC). The method adopted in this research was UV spectrophotometry and Fourier-transform infrared spectroscopy (FTIR) [8]. The sample composition impacts this property, potentially revealing what is in the sample and at what concentration. Fourier-transform infrared spectroscopy (FTIR) is a technique used to obtain an infrared spectrum of absorption or emission of a solid, liquid or gas. High-resolution spectral data are concurrently collected over a broad spectral range by a FTIR spectrometer. Compared to a dispersive instrument, FTIR spectroscopy offers a higher signal-tonoise ratio. Spectrum may be obtained rapidly and efficiently with FTIR. By employing FTIR, accurate results can be obtained without needing external calibration. Additionally, because it is a non-destructive technique, it is simple to distinguish between organic and inorganic substances.
Collection of Perindopril Tablets and Preparation of Perindopril Powders
The different brands of Perindopril tablets were purchased from different pharmacies in Sungai Petani, Kedah D.A., Malaysia. A total of 30 tablets were bought for each brand. These brands will be named A, B, C, D, E and F in this research. Their further description is recorded in Table 1. The standard perindopril powder was available in the laboratory of AIMST University. Each brand’s 20 perindopril tablets were weighed using the microbalance. The 20 perindopril tablets then proceeded to measure the thickness and diameter using an electronic vernier caliper and the average thickness and diameter were noted. The 20 perindopril tablets of each brand were crushed into powder with the help of a mortar and pestle. The powder was kept inside a re-sealable plastic bag to avoid the powder’s contact with moisture, which may cause the denaturation of the drug sample. The re-sealable plastic bag was labelled clearly with the name and the crushing date. These powders were kept in the desiccator. Different brands of perindopril tablets are laid down in Table 1. UV-Visible spectrophotometer Shimadzu UV-1800 was connected to the computer. This equipment includes a light source, monochromator, sample and reference cells, amplifier, detector, and recording devices such as a computer. The componentrs of UV-Visible spectrophotometer were Tungsten filament lamps, Monochromator: (light of a single wavelength), Sample and reference cells, Detector. The photomultiplier tube was commonly used as a detector in UV-Visible spectrophotometer.
Fourier Transform Infrared Spectroscopy (Ftir) Method
FTIR Spectrometer (Perkin Elmer Spectrum two) connected to the computer which is shown in Figure 2.
a. The source
• IR energy is emitted from a glowing black-body source, and
the beam passes through an aperture, which controls the amount
of energy.
b. The interferometer
• The IR beam enters the interferometer where “spectral encoding”
takes place. The interferometer uses a reference laser for
precise wavelength calibration. c. The sample:
• The IR beam enters the sample compartment, where it is
transmitted through or reflected off the sample’s surface; the
sample absorbs specific frequencies of energy that are uniquely
characteristic of the sample.
c. The detector:
• The beam finally passes to the detector for final measurement.
d. The computer:
• The signal is digitized, the FFT (Fast fourier transform)
along with calculation takes place, and the final infrared spectrum
is presented to the user.
Uniformity of Weight, Thickness and Diameter of Perindopril
Tablets<br>
1. Twenty tablets of each brand of perindopril were weighed
and recorded.
2. The percentage deviation of individual weight from the average
weight was determined using formula 1 as mentioned below:

3. The deviation of individual weight from the average weight should not exceed the limits given by British Pharmacopoeia (B.P.) as shown in Table 2.
Uniformity of thickness and diameter of Perindopril tablets
Twenty tablets of perindopril were tested for the diameter and thickness using an electronic vernier caliper. The values of the diameter, thickness, and hardness were recorded. Each deviation is calculated and the deviation of the individual unit from the mean diameter should not exceed ± 5% for tablets with a diameter of less than 12.5 and ± 3% for a diameter of 12.5 mm or more.
Preparation of Standrad Stock Solution:
1. 521.75 mg of Perindopril standard was accurately weighed
and transferred to a 25ml volumetric flask and is then dissolved
in methanol and added sufficient methanol to make
up the volume to 25 ml and sonicated.
2. The residue in the stock solution was removed by filtration.
3. The residue was placed on a glass plate and placed inside the
chamber for drying at room temperature.
4. The weight of the dried excipient was weighed and recorded.
5. The stock solution was used to prepare working solutions
prepared by diluting the stock solutions of Perindopril with
distilled water.
6. The weight of the residues after filtration is shown in Table 3.
Preparation of Working Standard Solutions
A volume of 1ml, 2ml, 4ml and 6ml of stock solutions of each brand of perindopril was pipetted into 100ml volumetric flasks and the volume was made up with distilled water to obtain working standard solutions in the concentration of 8μg/ml, 16μg/ml, 32μg/ml and 48μg/ml.
Determination of UV- Absorbance of Different Concentrations
The standard working solution of Perindopril was scanned in UVVi’s spectrophotometer from 500-190 nm at the λ max of 213 nm.
The percentage of purity was calculated by the following formula:

Disintegration Test for Perindopril Tablets
1. The disintegration test apparatus consists of a rack holding
6 glass or plastic tubes, each having a 10-mesh screen at its
bottom.
2. The tubes were raised and lowered at a fixed rate in a water
bath maintained at 37±2°C.
3. Six tablets were placed, one in each tube, along with a plastic
disk over each tablet for each cycle.
4. The tubes were allowed to move up and down, and the time
required for each tablet to disintegrate and pass through the
screen is recorded.
Fourier Transform Infrared Spectroscopy (Ftir) Method
1. Before placing the sample material, the crystal plate was
cleaned with distilled water, followed by methanol.
2. Solid samples of perindopril were positioned flat on the crystal
plate and clamped down with a pressure arm.
3. Pressure applied to the crystal plate was adjusted using a
pressure arm to ensure consistent contact between the crystal
and the sample.
4. Started scanning samples using spectrum software.
5. The data were analysed and the results were reported.
Average Weight of Different Brands of Perindopril Tablets
The average weight of different brands of perindopril is shown in Table 4. The table shows that brand A has the highest weight (104.35mg) per tablet, while brand B has the lowest weight (91.625 mg) per tablet. The average weight for Brands C, D, E, and F were 91.69 mg, 100.16 mg, 90.145 mg, and 92.43 mg per tablet, respectively. Uniformity of comparative weight variation of Perindopril tablets is shown in Table 5. According to Table 5, Brands A, B, C, D, E and F have an average weight of between 80 mg to 250 mg. According to the average weight given by British Pharmacopoeia (B.P.) shown in Table 2, the tablets for Brand A, B, C, D, E and F did not exceed the limits, which is a minimum of 18 of the tablets are within 7.5% and maximum 2 tablets have deviation of within 15.0%. Therefore, Brand A, B, C, D, E and F have passed the test. Average diameter and thickness of different brands of Perindopril tablets is laid down in Table 6. The average diameter and thickness of different brands of perindopril are shown in Table 6. The table shows that brand A has the highest diameter of 8.54 mm while brand D has the lowest diameter of 6.44 mm. The average diameter for Brands B, C, E and F was 8.12 mm, 6.58 mm, 8.11 mm, and 8.10 mm per tablet, respectively. The table shows that brand C has the highest thickness, 3.27 mm, while brand F has the lowest thickness, which is 2.71 mm. The average thickness for Brands A, B, D and E are 3.06 mm, 2.76 mm, 2.92 mm, and 2.73 mm per tablet, respectively. Uniformity of Diameter and Thickness of Different Brands of Perindopril tablets is shown in Table 7.
Note: D: Diameter T: Thickness
The average diameter of Brand A, B, C, D, E and F is less than 12.5mm. Each deviation is calculated, and the deviation of the individual unit from the mean diameter should not exceed ± 5% for tablets with a diameter of less than 12.5. According to Table 8, Brand A, B, C, D, E and F did not exceed the limits as the deviation of individual units from the mean diameter did not exceed ± 5%. The absorbance of different brands of perindopril is laid down in Table 8. UV-absorbance of different brands of perindopril at a concentration is shown in Graphs 1-5 and accumulated curves are laid down in Graph 6. The first, second and third readings of 8 μg/mL standard perindopril were 0.186, 0.187 and 0.187. The average for the standard was 0.187. The first, second and third readings of 8 μg/mL of Brand B were 0.186, 0.181 and 0.183. The average for brand B was 0.183. Besides, the first, second and third readings of 8 μg/mL of Brand C were 0.180, 0.184 and 0.182. The average for brand C was 0.182. The first, second and third readings of 8 μg/mL of Brand D were 0.185, 0.184 and 0.184. The average for brand D was 0.184. The first, second and third readings of 8 μg/mL of Brand E were 0.168, 0.168 and 0.169. The average for brand E was 0.168. Lastly, the first, second and third readings of 8 μg/mL of Brand F were 0.164, 0.161 and 0.161. The average for brand F was 0.162. It was found that the standard/Brand A has the highest average absorbance of 0.187, and Brand F has the lowest absorbance of 0.162 at the concentration of 8 μg/mL. The first, second and third readings of 16 μg/mL standard perindopril were 0.399, 0.4 and 0.4.
The average for the standard was 0.4. The first, second and third readings of 16 μg/mL of Brand B were 0.397, 0.400 and 0.398. The average for brand B was 0.398. Besides, the first, second and third readings of 16 μg/mL of Brand C were 0.372, 0.372 and 0.371. The average for brand C was 0.372. The first, second and third readings of 16 μg/mL of Brand D were 0.399, 0.401 and 0.399. The average for brand D was 0.400. The first, second and third readings of 16 μg/ mL of Brand E were 0.366, 0.353 and 0.360. The average for brand E was 0.359. Lastly, the first, second and third readings of 16 μg/mL of Brand F were 0.355, 0.345 and 0.355. The average for brand F was 0.352. It was found that the standard/Brand A and Brand D have the highest average absorbance of 0.4, and Brand F has the lowest absorbance of 0.352 at the concentration of 16 μg/mL.
The first, second and third readings of 32 μg/mL standard perindopril were 0.787, 0.788 and 0.790. The average for the standard was 0.788. The first, second and third readings of 32 μg/mL of Brand B were 0.789, 0.780 and 0.785. The average for brand B was 0.785. Besides, the first, second and third readings of 32 μg/mL of Brand C were 0.713, 0.708 and 0.710. The average for brand C was 0.710. The first, second and third readings of 32 μg/mL of Brand D were 0.786, 0.787 and 0.789. The average for brand D was 0.787. The first, second and third readings of 32 μg/mL of Brand E were 0.683, 0.664 and 0.676. The average for brand E was 0.674. Lastly, the first, second and third readings of 32 μg/mL of Brand F were 0.683, 0.679 and 0.679. The average for brand F was 0.680. It was found that the standard has the highest average absorbance of 0.788, and Brand E has the lowest absorbance of 0.674 at the concentration of 32 μg/mL.
The first, second and third readings of 48 μg/mL standard perindopril were 1.159, 1.156 and 1.165. The average for the standard was 1.160. The first, second and third readings of 48 μg/mL of Brand B were 1.111, 1.100 and 1.105. The average for brand B was 1.105. Besides, the first, second and third readings of 48 μg/mL of Brand C were 1.160, 1.156 and 1.158. The average for brand C was 1.158. The first, second and third readings of 48 μg/mL of Brand D were 1.160, 1.147 and 1.159. The average for brand D was 1.155. The first, second and third readings of 48 μg/mL of Brand E were 1.069, 1.051 and 1.059. The average for brand E was 1.06. Lastly, the first, second and third readings of 48 μg/mL of Brand F were 1.020, 1.023 and 1.021. The average for brand F was 1.021. It was found that the standard has the highest average absorbance of 1.160, and Brand F has the lowest absorbance of 1.021 at the concentration of 48 μg/mL.
Comparison by UV-Absorbance of Different Brands of Perindopril at Different Concentrations
The reading of 8 μg/ml, 16 μg/ml, 32 μg/ml, and 48 μg/ml of standard perindopril were 0.187, 0.400, 0.788 and 1.16. The reading of 8 μg/ml, 16 μg/ml, 32 μg/ml, and 48 μg/ml of Brand B were 0.183, 0.398, 0.785 and 1.105. The reading of 8 μg/ml, 16 μg/ml, 32 μg/ml, and 48 μg/ml of Brand C were 0.182, 0.372, 0.710 and 1.158. The reading of 8 μg/ml, 16 μg/ml, 32 μg/ml, and 48 μg/ml of Brand D were 0.184, 0.400, 0.787 and 1.155. The reading of 8 μg/ml, 16 μg/ ml, 32 μg/ml, and 48 μg/ml of Brand E were 0.168, 0.359, 0.674 and 1.060. The reading of 8 μg/ml, 16 μg/ml, 32 μg/ml, and 48 μg/ml of Brand F were 0.162, 0.352, 0.680 and 1.021.
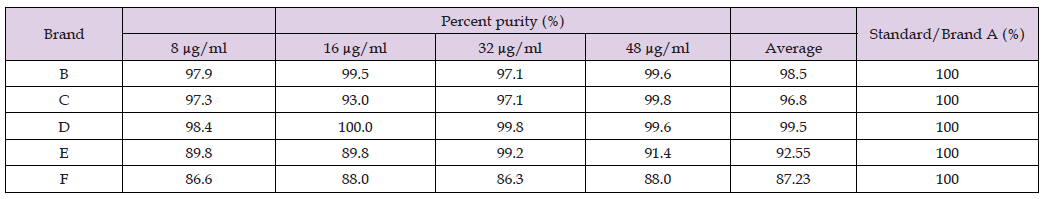
Table 9: Percent purity Determination of Different Brands of Perindopril by using the UV Spectrophotometric method.
Accumulated Calibration Curves of Different Brands of Perindopril
The correlation coefficient of an acceptable calibration is 0.9 or better. It was observed that all of the brands have a correlation coefficient of 0.9 and above. The correlation coefficient of standard/ Brand A was 0.9994; Brand B was 0.9965; Brands C and D were 0.9992; Brand E was 0.9981, and Brand F was 0.9995. Percent purity Determination of Different Brands of Perindopril by using the UV Spectrophotometric method is laid down in Table 9. The percent purity of standard perindopril was 100 %. There was a total of 4 concentrations conducted for each assay of the sample. The percent purity of 8 μg/ml, 16 μg/ml, 32 μg/ml, and 48 μg/ml of Standard/ Brand A were 100 %. The average percent purity of sample A was 100 %. The percent purity of 8 μg/ml, 16 μg/ml, 32 μg/ml and 48 μg/ml of Brand B were 97.9%, 99.5%, 97.1% and 99.6%. The average percent purity of sample B was 98.5 %. The percent purity of 8 μg/ml, 16 μg/ml, 32 μg/ ml and 48 μg/ml of Brand C were 97.3%, 93.0 %, 97.1 % and 99.8%. The average percent purity of Brand C was 96.8 %. The percent purity of 8 μg/ml, 16 μg/ml, 32 μg/ml and 48 μg/ml of Brand D were 98.4 %, 100 %, 99.8% and 99.6 %. The average percent purity of Brand D was 99.5 %. The percent purity of 8 μg/ml, 16 μg/ml, 32 μg/ml and 48 μg/ ml of Brand E were 89.8 %, 89.8 %, 99.2 and 91.4 %. The average percent purity of Brand E was 92.55 %. The percent purity of 8 μg/ml, 16 μg/ml, 32 μg/ml and 48 μg/ml of Brand F were 86.6 %, 88.0 %,86.3 % and 88.0 %. The average percent purity of Brand F was 87.23 %. Comparison of Percent Purity between Standard and Sample by Using UV Spectrophotometric Method is given in Graph 7.
Comparison of percent purity between the standard and sample by using the UV Spectrophotometric method is recorded in Graph 7. The percent purity of standard/ Brand A perindopril was 100 %. Besides, the average percent purity of Brands B, C, D, E, and F were 98.5 %, 96.8 %, 99.5 %, 92.55 %, and 87.23 %. Brand D has the highest percentage of purity, while Brand F has the lowest percentage of purity compared to Standard/Brand A. According to British Pharmacopoeia (BP), the content of perindopril in tablets should be in the range of 92.5 % to 105.0 %. Thus, Brand A, B, C, D and E passed the test, and Brand F failed the test. Disintegration / time test of different brands of perindopril tablets is shown in Table 10. The disintegration time of different brands of perindopril was recorded in Table 10 and Graph 8. Brand F took the longest time to disintegrate, which is 8.43 minutes, while Standard/ Brand A took the shortest time to disintegrate, which is 0.7 minutes. The time taken for tablets to disintegrate for Brand B, Brand C, Brand D and Brand E were 5.97 minutes, 2.78 minutes, 4.13 minutes, and 1.35 minutes. According to USP limits for disintegration time for tablets, the time taken for uncoated tablets to disintegrate in water at 37°C ± 2°C is 30 minutes 8. It was found that all the tablets disintegrate in 30 minutes. Therefore, all of the Brands of perindopril taken passed the disintegration test. FTIR spectrum of different brands of Perindopril Tablets are shown in Graphs 9-14 respectively and comparison in Graph 15.
1. FTIR principal peaks corresponding to the structural features
of Perindopril are shown in Graph 9. Wave number 2898.63
cm-1 disclosed the presence of C-H stretching, and wave number
1671.48 cm-1 showed the presence of C=O stretching. Besides
that, the broad peak at 2500 -3000 cm-1 and peak at
1260.45 cm-1 confirm the acid group and a further peak at
3262.29 cm-1 with 1640 cm-1 confirms the presence of amine
with amide linkage. Four absorptions at 1450-1650 cm-1 indicated
the presence of C=C aromatic. Moreover, medium absorption
near 1000-1300 cm-1 confirms the presence of the
ester group.
2. FTIR principal peaks corresponding to the structural features
of Perindopril are shown in Graph 10. Wave number
2898.77 cm-1 disclosed the presence of C-H stretching, and
wave number 1624.14 cm-1 showed the presence of C=O
stretching. Besides that, the broad peak at 2500 -3000 cm-1
and peak at 1259.81 cm-1 confirm the acid group and a further
peak at 3261.93 cm-1 with 1640 cm-1 confirms the presence
of amine with amide linkage. Four absorptions at 1450-
1650 cm-1 indicated the presence of C=C aromatic. Moreover,
medium absorption near 1000-1300 cm-1 confirms the presence
of the ester group.
3. FTIR principal peaks corresponding to the structural features
of Perindopril are shown in Graph 11. Wave number
2916.18 cm-1 disclosed the presence of C-H stretching, and
wave number 1617.94 cm-1 showed the presence of C=O
stretching. Besides that, the broad peak at 2500-3000 cm-1
and peck at 1154.38 cm-1 confirm the acid group and a further
peak at 3336.27 cm-1 with 1640 cm-1 confirms the presence
of amine with amide linkage. Four absorptions at 1450-
1650 cm-1 indicated the presence of C=C aromatic. Moreover,
medium absorption near 1000-1300 cm-1 confirms the presence
of the ester group.
4. FTIR principal peaks corresponding to the structural features
of Perindopril are shown in Graph 12. Wave number
2900.64 cm-1 disclosed the presence of C-H stretching, and
wave number 1641.39 cm-1 showed the presence of C=O
stretching. Besides that, the broad peak at 2500-3000 cm-1
and peck at 1251.64 cm-1 confirm the acid group and a further
peak at 3282.67 cm-1 with 1640 cm-1 confirms the presence
of amine with amide linkage. Four absorptions at 1450-
1650 cm-1 indicated the presence of C=C aromatic. Moreover,
medium absorption near 1000-1300 cm-1 confirms the presence
of the ester group.
5. FTIR principal peaks corresponding to the structural features
of Perindopril are shown in Graph 13. Wave number
2898.96 cm-1 disclosed the presence of C-H stretching, and
wave number 1641.54 cm-1 showed the presence of C=O
stretching. Besides that, the broad peak at 2500-3000 cm-1
and peck at 1259.59 cm-1 confirm the acid group and a further
peak at 3267.15 cm-1 with 1640 cm-1 confirms the presence
of amine with amide linkage. Four absorptions at 1450-
1650 cm-1 indicated the presence of C=C aromatic. Moreover,
medium absorption near 1000-1300 cm-1 confirms the presence
of the ester group.
6. FTIR principal peaks corresponding to the structural features
of Perindopril are shown in Graph 14. Wave number
2898.97 cm-1 disclosed the presence of C-H stretching, and
wave number 1641.60 cm-1 showed the presence of C=O
stretching. Besides that, the broad peak at 2500-3000 cm-1
and peck at 1259.69 cm-1 confirm the acid group and a further
peak at 3327.37 cm-1 with 1640 cm-1 confirms the presence
of amine with amide linkage. Four absorptions at 1450-
1650 cm-1 indicated the presence of C=C aromatic. Moreover,
medium absorption near 1000-1300 cm-1 confirms the presence
of the ester group.
Comparison of FTIR Spectrum of different brands of Perindopril.
All the brands of perindopril obtained were found to have structural characteristics of perindopril. Saturated alkane absorptions were observed on the right side of 3000 cm-1 in every brand. The wave number between 1600-1900 cm-1 showed the presence of C=O stretching. Besides that, the broad peak at 2500-3000 cm-1 and peck at 1000-1300 cm-1 confirm the acid group. The acid group is confirmed at the peak around 3600 cm-1. Furthermore, medium absorption of N-H stretch near 3500 cm-1 was observed, and the presence of amine with amide linkage was confirmed at 1640 cm-1. Four absorptions at 1450-1650 cm-1 indicated the presence of C=C aromatic. Moreover, medium absorption near 1000-1300 cm1 confirms the presence of the ester group.
The objective of this research was to perform the quantitative determination of different brands of perindopril tablets available in Malaysia by using UV spectrophotometric and Fourier transform infrared spectroscopy methods. This research uses the quality control test, UV spectrophotometric assay, disintegration test, and stuctural characterization by FTIR method. The weight uniformity test of tablets represented the quality control of specific batches of tablets. The test was performed to check whether the tablets used in the assay of perindopril contained the amount of active pharmaceutical ingredient (API) intended, with little variation among tablets within a batch. During uniformity weight testing, 20 tablets of each brand of perindopril were weighed individually, and the average weight was calculated. The percentage of deviation of each brand of perindopril tablet was calculated, and it was found that tablets for Brand A, B, C, D and F did not exceed the limits given by British Pharmacopoeia, which is a minimum of 18 of the tablets are within 7.5% and maximum 2 tablets have deviation of within 15.0 %. Therefore, Brand A, B, C, D, E and F passed the weight uniformity test. In conclusion, all of the perindopril of each brand had uniform weights.
The UV spectrophotometric method was used to determine the percent purity of various perindopril brands. The procedure of this assay was provided by B. P. Spectrometer was used to measure the amount of ultraviolet radiation absorbed by perindopril in the sample solution. At the wavelength of maximum absorbance of 213 nm, the absorbance of the dilutions and standard preparation in a 1cm cell was measured using a spectrophotometer. The absorbances will be utilized for each sample to determine the percent purity. Filtration was involved in this method. This was because filtration can remove the undissolved excipients from the perindopril solution. Comparison of percent purity between the standard and sample by using the UV spectrophotometric method was recorded in Graph 7. According to B.P., the percent purity of perindopril in tablets should be in the range of 92.5 % to 105.0 %. The average percent purity of Brand A, B, C, D, E, and F were 100%, 98.5 %, 96.8 %, 99.5 %, 92.55 %, and 87.23 %. Brand D has the highest percentage of purity, while Brand F has the lowest percentage of purity compared to Standard/Brand A. Thus, Brand A, B, C, D and E passed the test, and Brand F failed the test. This indicated Brand B and Brand D were comparatively better than other brands used in this research. In conclusion, Brand B, C, D and E were within the limit specified by B.P. Brand D was the best among these 6 perindopril tablet brands in this assay of perindopril by UV spectrophotometric method. Besides, disintegration time test is carried out to determine the time required for the tablet to disintegrate. The time taken for tablets to disintegrate for Brand B, Brand C, Brand D and Brand E were 5.97 minutes, 2.78 minutes, 4.13 minutes, and 1.35 minutes. According to USP limits for disintegration time for tablets, the time taken for uncoated tablets to disintegrate in water at 37°C ± 2°C is 30 minutes [8]. It was found that all the tablets disintegrate in 30 minutes. Therefore, all of the Brands of Perindopril passed the disintegration test.
Fourier-transform infrared spectroscopy (FTIR) is a technique that measures the range of wavelengths in the infrared region that are absorbed by a solid or liquid [9-15]. This is accomplished by applying infrared radiation (IR) to material samples. The IR spectrometer then measures the sample’s absorbance of the IR light’s energy at various wavelengths to determine the material’s molecular composition and structure. The IR spectrum is a graph of infrared light absorbance by the substance on the vertical axis and the frequency on the horizontal axis. According to the data obtained, all the brands of perindopril obtained were found to have the structural characteristics of perindopril. Saturated alkane absorptions were observed on the right side of 3000 cm-1 in every brand. The wave number between 1600- 1900 cm-1 showed the presence of C=O stretching. Besides that, the broad peak at 2500-3000 cm-1 and peck at 1000-1300 cm-1 confirm the acid group. The acid group is confirmed at the peak around 3600 cm-1. Furthermore, medium absorption of N-H stretch near 3500 cm-1 was observed, and the presence of amine with amide linkage was confirmed at 1640 cm-1. Four absorptions at 1450-1650 cm-1 indicated the presence of C=C aromatic [16-20]. Moreover, medium absorption near 1000-1300 cm-1 confirms the presence of the ester group.
In conclusion, Brand B and Brand D showed excellent results to fulfil all the protocol for quantitative and quantitative test among all brands of perindopril used in this research. Brand B, C, D and E have a percentage of purity within the standard provided by the British Pharmacopoeia. The Brand F has not qualified the standard provided by the British Pharmacopoeia. Therefore, Brand F should be considered as substandard tablets. It was concluded that the developed method was found to be simple, accurate and can be used for routine quality analysis of perindopril tablets. The assay of pharmaceutical products should always be performed to ensure the safety, quality, quantity, and efficacy of pharmaceutical products.
This research work was sponsored by the Faculty of Pharmacy, AIMST University, Malaysia, which is highly appreciated.
The authors declare that there is no conflict of interests.


