Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Yefei Wang1*, Beiying Wu2, Wenquan Xia1, Ning Chen1 and Yiqun Hu1
Received: July 20, 2023; Published: August 10, 2023
*Corresponding author: Yefei Wang, Faculty of Medical Laboratory Science, Ruijin Hospital, School of Medicine, Shanghai Jiao Tong University, 197 Ruijin Er road, shanghai 200025, China
DOI: 10.26717/BJSTR.2023.52.008205
Hemoglobinopathies are common inherited diseases in southern China. The aim of the present study is to analyze the hematologic and molecular features of hemoglobinopathies in Shanghai, in order to provide a reference data for screening hemoglobinopathies. A total of 1029 samples were studied using High Performance Liquid Chromatography (HPLC) on the Bio-Rad Variant II HPLC system. GAP-PCR and reverse dot blot (RDB) were used to detect globin gene mutation or deletion. DNA sequencings for Alpha Globin gene (HBA1/A2) and Beta Globin gene (HBB) were simultaneously performed. We found that among 1029 samples, beta-thalassemia was the predominant type of hemoglobinopathy (39.16%). Six (0.58%) cases of beta-thalassemia major and 397 (38.58%) cases of beta-thalassemia carriers were identified. Among those beta-thalassemia samples, a total of 19 mutations were found, among which the β654/βN mutation (168/1029,16.33%) was the main type, followed by the β41-42/βN mutation (118/1029,11.47%). There were 136 (13.22%) cases of alpha-thalassemia (silent carrier & minor) and 39 (3.79%) cases of HbH disease. A total of 10 gene mutations were found in the alpha-thalassemia samples, among which the main genotype of deletion α-thalassemia was --SEA /αα(119/1029,11.56%), while non-deletion α-thalassemia was uncommon in our report(2/1129,0.19%) both of which was αWSα/αα. The main genotype of HbH was --SEA /-α3.7 (28/1029, 2.72%). In 13 (1.26%) cases of αβ- thalassemia, with a total of 9 genotypes were found, among which the more common genotypes were -α3.7/ β654, --SEA/β41-42 and --SEA/β17. The ten main structural hemoglobin variants of 14 (1.36%) cases were Hb E, HbG-Taipei, Hb Q-Thailand, Hb Youngstown, Hb Guangzhou-Hangzhou, Hb M-Boston, Hb G-Siriraj, Hb J-Baltimore, Hb J-Sicilia and Hb Tamano. In addition, a single synonymous mutation (HBB c.9T>C, His>His) was found in 2 cases. Our results provide a detailed prevalence, hematologic and molecular characterization of hemoglobinopathies in Shanghai. We hope these findings will help to increase the awareness of this disease and provide useful epidemiological information for screening.
Keywords: Hemoglobinopathies; High Performance Liquid Chromatography; Globin Gene
Hemoglobinopathy is a type of genetic defect that results in abnormal structure or amount in one of the globin chains of the hemoglobin molecule, including thalassemias and structural hemoglobin variants (abnormal hemoglobins). The thalassemias are an autosomal recessively inherited group of disorders of hemoglobin synthesis characterized by the absence or reduction in output of one or more of the globin chains of hemoglobin. The structural variants result from substitution of one or more amino acids in any of the globin chains of the hemoglobin molecule, usually in α or β globin chain. The substitution of amino acids in most of the Hb variants so far doesn’t result in an abnormal stability and dysfunction of the hemoglobin molecule, and they are usually clinically silent or nonsignificant. Significant symptoms such as anemia may exist if it is present along with thalassemia or other disorders. The key element in the diagnosis of hemoglobinopathies is laboratory findings.
More than one thousand hemoglobin variants have been identified regarding changes in the globin chains [1]. Hemoglobinopathies have a wide geographical distribution [2]. In China, obvious ethnic and regional differences in hemoglobinopathies exist. There is a high incidence of α- or β-thalassemias in southern China, especially in Guangdong, Guangxi and Hainan provinces [3]. More than 80 structural Hb variants have been reported so far, among which Hb E is the most common one in Yunnan, the province with the highest morbidity of abnormal hemoglobin. Hb G/ D is more common in northern China while Hb E / J is more common in southern China [3]. As an international metropolitan city, population migration is also more common in Shanghai, which leads to the migration of diseases. However, there have been few epidemiological studies of hemoglobinopathies in Shanghai. We analyzed the Hb variants screened by HPLC, comparing with their properties in the globin gene detection to investigate the hematologic and molecular characteristics in local population, and to provide epidemiological data related to structural Hb variants.
Subjects
A Total 1029 cases were investigated in our laboratory during the study period from Nov.2014 to Dec.2018. This population included patients with clinical suspicion of hemoglobinopathies and those individuals’ presenting hemolysis who were screened for the presence of hemoglobinopathies in Ruijin Hospital affiliated to Shanghai Jiao Tong University School of Medicine. The following combination of tests were used in hemoglobinopathy screening. (Figure 1).
Methods
Hematologic Routine Tests: Complete blood count and red blood cell indices (Hb, MCV, MCH, MCHC) were measured by the automated cell analyzer (SYSMEX XE-2100). Wright-stained peripheral blood smears were examined for red blood cell morphology.
Hemoglobin Analysis: 2ml of whole venous blood were collected in a vacuum blood collection tube containing EDTA as an anti-coagulent. HbA2, HbF, and other hemoglobin variants were analyzed using HPLC method used for chromatographic separation of human hemoglobin on the Variant Hemoglobin Testing System (Variant II Beta Thalassemia Short Program, Bio-Rad Laboratories). No preparation was required unless the sample was collected from severe anemic patients or there was less than 500 𝜇L of sample in the tube. In such case, sample was manually prediluted by mixing 1.0mL wash/diluents with 5 𝜇L of whole blood sample. HbA2/F calibrators and normal and abnormal controls were analyzed at the beginning of each run. Reports and chromatograms generated were studied and interpreted by observing HbA2 and F concentration for beta thalassemia and retention time and area percentage of other peaks and windows for structural variants. Each chromatogram shows peaks of HbA0, A2, and HbF along with C, D and S window, and two minor peaks, P2 and P3.
Globin Gene Analysis: Molecular analyses for common alpha deletions and common beta mutations were performed by using GAP-PCR and reverse dot blot (RDB). Full DNA sequencing of Alpha Globin (HBA1/A2) and Beta Globin (HBB) genes was simultaneously performed in individuals with an abnormal HPLC results but a negative thalassemia gene screening.
Statistical Method
Data entry and analysis were performed using the Statistical Package for Social Sciences (SPSS21.0). P<0.05 was considered statistically different.
Hematological Findings
Means of RBC Parameters and HbA2: Comparison of all groups is shown in Table 1. In our studies, 439 subjects had HbA2 levels > 3.5%, in which 423 (96.4%) cases were identified as β-thalassemia carriers. αβ- thalassemia and structural Hb variants by globin gene analysis (HbA2 range 3.6%~72.7%). 40 subjects had an increased area at retention times of 0.125±0.021min and 0.406±0.091min, among which 39 (97.5%) subjects were identified to have HbH disease by globin gene analysis (HbA2 range 0%~ 2.1%) (Figure 2). A significant (p<0.05) difference in HbA2 was found in each comparison group, except for those between the gene negative group and the α- thalassemia silent carrier and minor groups(p=0.421>0.05, and between theβ- thalassemia and theαβ- thalassemia group (p=0.572>0.05. A significant (p<0.05) difference of RBC was found in the gene negative group compared with all other groups. A significant (p<0.05) difference of MCV was found in the gene negative group compared with the α-, β-,αβ- thalassemia or Hb variants groups (Table 1) (Figure 3).
RBC Parameters and Morphology of Structural Hemoglobin Variants: Comparison of all hemoglobin variants is shown in Table 2.
Table 2: RBC parameters and morphology features of 16 structural hemoglobin variants or gene mutation.
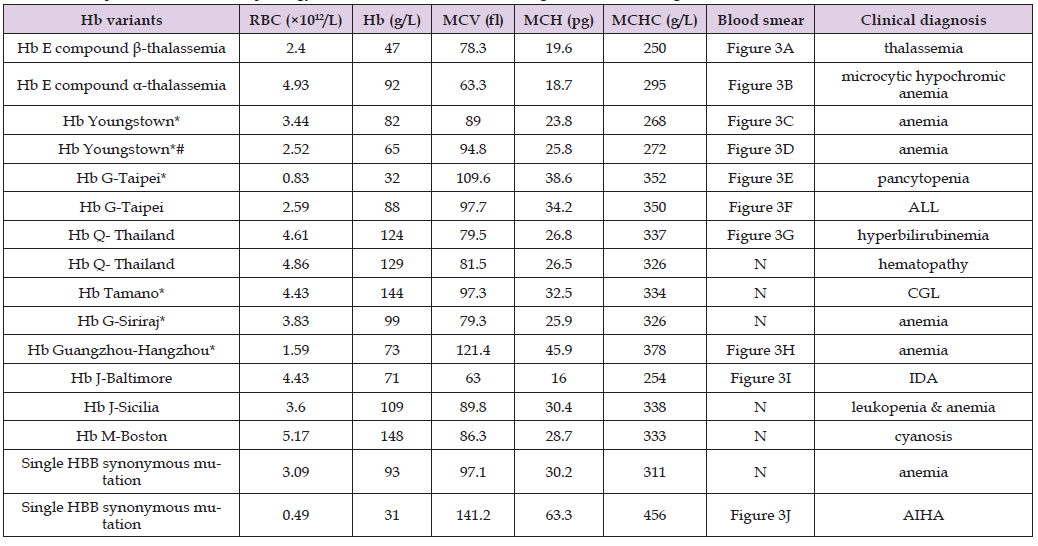
Note: *compound HBB c.9T>C synonymous mutation; #compound HBA2 c.382A>T[127Lys> termination codon; ALL: acute lymphocytic leukemia; CGL: chronic granulocytic leukemia; IDA: iron
Hemoglobin Analysis of Structural Hemoglobin Variants
Ten structural hemoglobin variants were detected in 14 cases (14/1029,1.36%). Among those 4 cases were shown in A2 window (1 Hb E compound β-thalassemia, 1 Hb E compound α-thalassemia and 2 HbG-Taipei), 6 cases were shown in S window (2 cases of Hb Q-Thailand, 2 cases of Hb Youngstown, 1 case of Hb Guangzhou-Hangzhou, and 1 case of Hb M-Boston), 1 case was shown in C window which is Hb G-Siriraj, 2 cases were shown in P3 window with one Hb J-Baltimore and one Hb J-Sicilia. One case of Hb Tamano was shown between A0 and A2 window (Figure 4). Another 2 cases were shown in C window (Figure 5) which were proved to be a single synonymous mutation by DNA sequencing (HBB c.9T>C, His>His) without abnormal Hb variant. This kind of synonymous mutation was also found in most of the Hb variants above. Presumptive identification of hemoglobin variants was made primarily using retention time (RT) windows and area percent (Table 3).
Table 3: RBC parameters and morphology features of 16 structural hemoglobin variants or gene mutation.
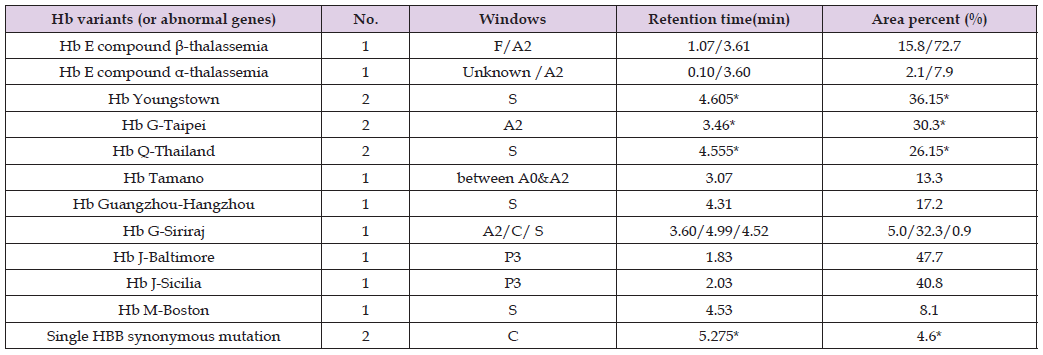
Notes: *shown in means.
Globin Gene Analysis
In our studies, a total of 605 subjects were found to be hemoglobinopathies by globin gene analyzing (605/1029, 58.79%), in which β-thalassemia (403/1029, 39.16%) was more common than other hemoglobinopathies. The common mutations in our study population include --SEA/αα(119/1029,11.56%) in α-thalassemia ,and CD41/42(- TTCT) (118/1029,11.47%) or IVS-2-654(C→T)(168/1029, 16.33%) in β-thalassemia (Table 4). The DNA sequencing results of 10 types of hemoglobin variants are shown in Figure 6, in which βglobin gene mutation was more common (9/14, 64.3%), shown in Table 5.
Hemoglobinopathy is a common inherited single gene disorder in southern China. Many studies have been published from southern China on hemoglobinopathies; however most studies focus on epidemiology and screening. While very few studies did extensive analysis on thalassemic and structural Hb variants carriers determined using HPLC reports in Shanghai, an international metropolitan city with frequent population migration that inevitably leads to the migration of diseases. The most common conventional method of screening for any inherited blood disorder is clinical examination followed by CBC, electrophoresis or HPLC [4-7] . Out of these, HPLC method is based on the movement of different hemoglobins in a given gravitational field as they pass through certain adhesive materials. HPLC has the advantage of quantifying HbF and HbA2 along with detecting other variants in a single screening test [8]. Automated HPLC and β-thalassemia program is an appropriate approach for the screening and presumptive identification of patients as well as carrier of β-thalassemia prior to DNA studies for definitive diagnosis.
As the first laboratory to analyze hemoglobin by using the automated HPLC and β-thalassemia program in Shanghai. 39 (3.79%) HbH disease,403 (39.16%)β-thalassemia, 13 (1.26%)αβ-thalassemia and 14 (1.36%) cases of structural Hb variants had been identified in hemoglobinopathy screening, which were then confirmed to be hemoglobinopathies by globin gene analyzing and confirmation on the the Hbvar database (http://globin.cse.psu.edu/hbvar/menu.html). The automated HPLC method showed a high sensitivity and specificity in β-thalassemia, αβ-thalassemia, HbH disease and structural Hb variants screening, but displayed a low sensitivity in detecting α-thalassemia silent carrier or minor type. A total of 6 (0.58%) cases of β-thalassemia major and 397 (38.58%) cases of β-thalassemia carriers, 136 (13.22%) cases of α-thalassemia (silent carrier & minor ), 39 (3.79%) cases of HbH disease, 13 (1.26%) cases of αβ- thalassemia were confirmed by gene analyzing. Among them, the common genotypes of deletion α-thalassemia were --SEA /αα, -α3.7/ααand -α4.2/αα. --SEA /-α3.7, --SEA /-α4.2, --SEA /αCS αwere of HbH diease. The main genotypes of deletion and non-deletion α-thalassemia were --SEA/αα and αWSα/αα. The main genotypes of β-thalassemia wereβ654/βN followed byβ41-42/βN which was different from the results published from southern China [9-14]. Therefore there is a significant geographical difference in the thalassemia gene mutation prevalence which constitutes the diversity of HBA and HBB mutations.
The ten types of structural Hb variants we found were Hb E compound with thalassemia, HbG-Taipei, Hb Q-Thailand, Hb Youngstown, Hb Guangzhou-Hangzhou, Hb M-Boston, Hb G-Siriraj, Hb J-Baltimore, Hb J-Sicilia and Hb Tamano. In comparison, HbE and Hb Q-Thailand are common in southern china. While HbG-Taipei has been reported in both Han and other national minority in Chinese population [15], all other variants are rare. Hb J-Baltimore (β16 Gly→Asp) was first described in1963 in an African-American family. Since then, several cases have been reported in distinct racial groups. Most of those cases were discovered incidentally during the study of other entities, such as thalassemia [16]. Hb J-Sicilia (β65 lys→Asn) was first described in1974 in a young Sicilian woman. This Hb variant doesn’t show any difference in function, which can be considered as a homologue of Hb Zambia (β60 lys→Asn [17]. Hb J-Baltimore and Hb J-Sicilia were the first to be reported in Chinese population, both of which have compound homozygous mutation in HbA2 gene 5’- UTR and a synonymous mutation (c.9T>C) in HBB gene. Whether those mutations cause a change in Hb function needs further research. The heterozygous carrier of Hb J-Baltimore seen in a 37-year-old woman who displayed a moderate microcytic hypochromic anemia with iron deficiency. The case of Hb J-Sicilia was seen in a 61-year-old woman who suffered from leukopenia and slight anemia. In our studies, except for 2 cases of HbE with compound thalassemia and 2 cases of Hb Youngstown display marked hemolysis and anemia and one case of HbM-Boston displays characteristic cyanosis, most of structural Hb variants which were without any clinical effects and were fortuitously found during screening programmes.
The majority of Hb variants fortuitously discovered are of minimal clinical interest. On the contrary, those found during the course of a hematological disorder reveal the aetiological answer for the disease. Often time, unusual clinical presentations may be explained by the presence of several Hb abnormalities and their identification may require further investigations [18]. Many of these variants are of little clinical significance in heterozygous state, but when combined with other variants or disease they may give rise to severe disease. Therefore, in those patients once the Hb variants have been screened by HPLC, genetic analysis is recommended and the clinical characteristics of the disease should be communicated with patients to avoid serious hemolysis complications. Significant (p<0.05) differences in HbA2 were found in each group, except for those between the gene negative group and the α- thalassemia silent carrier and minor group(p>0.05. Significant(p<0.05)differences of both RBC and MCV were found in the gene negative group compared withα-, β-,αβ- thalassemia or Hb variants groups. Hence, it is of great value to screen hemoglobinopathies except α- thalassemia silent carrier and minor by detecting RBC, MCV, and HbA2. For those samples with increased RBC and decreased MCV but normal HbA2, further gene analysis should be followed to avoid misdiagnosing of α- thalassemia silent carrier and minor.
Our results provide a detailed prevalence, hematologic and molecular characterization of hemoglobinopathies in Shanghai that can definitely help to increase the awareness of hemoglobinopathy screening. β-thalassemia was the predominant type of hemoglobinopathies, with the main mutation being β654/βN followed byβ41-42/ βN. The main genotype of deletionα-thalassemia was --SEA /αα, and non-deletion α-thalassemia was uncommon in our report. The main genotype of HbH was --SEA /-α3.7. The more common genotypes of αβ- thalassemia were -α3.7/ β654, --SEA/β41-42 and --SEA/β17. There are 10 Hb variants in this study focusing on the population in Shanghai. HbE compound with thalassemia and Hb Youngstown presented with marked hemolysis and anemia, HbM-Boston showed characteristic cyanosis, while the other 8 types of Hb variants were clinically insignificant. We identified a rare case of Hb J-Baltimore (β16 Gly→Asp) and a rare case of Hb J-Sicilia (β65 lys→Asn) in Chinese population for the first time, both of which were compounded with a homozygous mutation in HbA2 gene 5’- UTR and a synonymous mutation( c.9T>C) in HBB gene.
No potential conflicts of interest were disclosed.
• Conception and Design: YF. Wang, BY. Wu, YQ. Hu.
• Acquisition of Data: (Provided reagents, acquired and managed
patients, provided facilities, etc.): YF. Wang, BY. Wu, WQ. Xia,
N. Chen.
• Analysis and Interpretation of Data: (e.g., Statistical analysis,
biostatistics, computational analysis): YF. Wang, BY. Wu, WQ. Xia.
• Writing, Review, and/or Revision of the Manuscript: YF.
Wang, BY. Wu.
• Administrative, Technical, or Material Support: (i.e., reporting
or organizing data, constructing databases): YF. Wang, YQ. Hu.
We are grateful to the staff at the Faculty of Medical Laboratory Science and Department of Clinical Laboratory at the Shanghai Jiao Tong University Affiliated Ruijin Hospital for the collection of samples from the related patients.
There are no financial conflicts of interest to disclose.


