Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Gao Zhen1#, Ba Ming Chuan2#, Liu Hao2, Su Zi Ce3, Li Li Jun2, Guo Lin2, Cai Fu Sheng2, Li Min Fei2, Xu Pei Yuan2, Zha Jie1, Wu Chang Fang1 and Zhang Hong Zhe1,2*
Received: July 18, 2023; Published: July 28, 2023
*Corresponding author: Zhang Hong Zhe, Department of Cardiology, The Seventh Affiliated Hospital, Southern Medical University, Desheng Road section 28, Foshan 528200, China
DOI: 10.26717/BJSTR.2023.51.008168
Objective: To explore the effect of different antiplatelet regimens on the incidence of hyperuricemia in patients with coronary atherosclerotic heart disease (Coronary Artery Disease, CAD).
Methods: A total of 326 patients diagnosed with coronary atherosclerotic heart disease (CAD) in the Department of Cardiology, The Seventh Affiliated Hospital of Southern Medical University from March 2021 to December 2021 were retrospectively analyzed. 214 cases of patients. After admission, they were divided into aspirin single-drug group (group A) with 22 cases, clopidogrel single-drug group (group C) with 24 cases, and aspirin combined with clopidogrel group (group AC) with 79 cases according to the random number table method according to different antiplatelet treatment regimens. For example, aspirin and ticagrelor combined group (AT group) 89 cases.
Results: In the comparison of an antiplatelet drug treatment regimen, the clopidogrel group (group C) was compared with the aspirin group (group A), and the incidence of hyperuricemia at maintenance dose was reduced by 25% (OR=0.25, 95%CI: 0.07-0.89, P=0.03); in the comparison of two platelet drug groups: aspirin combined with ticagrelor group (AT group) compared with aspirin combined with clopidogrel group (AC group), AT group maintained The incidence of hyperuricemia significantly increased at the lower dose (OR=2.02, 95%CI: 1.08-3.79, p=0.03). After CAD patients received antiplatelet therapy, their blood uric acid levels changed significantly, and this change became more and more obvious as the treatment continued.
Conclusion: In the treatment of CAD patients, when antiplatelet drugs are used alone, the incidence of hyperuricemia in the clopidogrel treatment group is significantly lower than that in the aspirin treatment group; when dual antiplatelet drugs are used, the combination of aspirin and clopidogrel The incidence of hyperuricemia in the treatment group was significantly lower than that in the combination use of aspirin and ticagrelor. Therefore, when choosing an antiplatelet regimen for CAD patients, the antiplatelet regimen that is more suitable for them should be selected according to their basal uric acid value. Finally, when using aspirin and dual antiplatelet regimens to treat blood uric acid levels, clinicians should pay attention to the management of blood uric acid levels, and recommend patients to review blood uric acid levels after 3 months of treatment, so as to adjust the drug regimen in time and carry out early intervention.
Keywords: Coronary Heart Disease; Hyperuricemia; Aspirin; Clopidogrel; Ticagrelor
Abbreviations: ACEI: ACE Inhibitor; ACS: Acute Coronary Syndrome; ADP: Adenosine Diphosphate; ARB: Angiotensin II Receptor Antagonist; AF: Atrial Fibrillation; CCB: Calcium Channel Blocker; CHD: Coronary Heart Disease; CIS: Chronic Ischemic Syndrome; DAPT: Dual Anti-Platelet Therapy; HRPR: High Residual Platelet Reactivity; HF: Heart Failure; HUA: Hyperuricemia; Protein-Coupled 12; PCI: Percutaneous Coronary Intervention; PLATO: Platelet Inhibition and Patient Outcomes; NSTEMI: Non-ST Elevation Myocardial Infarction; SA: Stable Angina; SAPT: Single Anti-Platelet Therapy; STEMI: ST-Segment Elevation Myocardial Infarction; SUA: Serum Uric Acid; UA: Unstable Angina
Uric acid is a crucial metabolic product that promotes purine oxidation and reduction, leading to elevated uric acid levels in the blood, which is termed hyperuricemia. Hyperuricemia is diagnosed if the blood uric acid level exceeds 7.0 m/dL (men) or 6.0 mg/dL (women). Hyperuricemia has become a serious global health problem as it leads to deterioration of health conditions. Epidemiological studies have shown that the incidence of hyperuricemia is increasing. The incidence of hyperuricemia is between 5% and 10% in the United States, while the incidence is as high as 26.1% in men and 17.1% in Asian countries [1]. According to the latest survey, more than 120 million cases of hyperuricemia have been reported in China, and the number of cases is increasing each year. Studies have shown that the increase in blood uric acid levels may be caused by various reasons, including age, gender, lipid levels, insulin resistance, diabetes, gout, and other metabolic syndromes, which can act as risk factors [2]. According to latest research, hyperuricemia can cause serious health problems, including high blood pressure and cardiovascular disease (such as ischemic heart disease and heart failure), and therefore, effective preventive measures [3] against hyperuricemia should be taken. Coronary atherosclerotic heart disease refers to a group of diseases in which atherosclerosis occurs in the coronary arteries (arteries), causing narrowing or occlusion of the arteries’ lumens, thereby leading to ischemia, hypoxia, or necrosis of the heart muscle, that is, coronary artery disease (CAD). Based on their characteristics and treatment strategies, diseases have recently been classified as follows: chronic ischemic syndrome (CIS) and acute coronary syndrome. CIS includes stable angina (SA), ischemic cardiomyopathy, and occult heart disease; unstable angina (UA), non-ST elevation myocardial infarction (non-ST elevation myocardial infarction NSTEMI), and ST-segment elevation myocardial infarction (STEMI) [4].
For SA patients, according to the 2020 edition of the Chinese Medical Association “Guidelines for the Primary Diagnosis and Treatment of Stable Coronary Heart Disease,” long-term oral administration of aspirin is recommended if aspirin contraindications are absent. Clopidogrel can be used for those with contraindications or who cannot tolerate aspirin. According to the “2020ESC Guidelines for Non-ST Elevation Acute coronary Syndrome,” for patients receiving percutaneous coronary intervention (PCI), P2Y12 antagonists are recommended for 12 months along with aspirin therapy to obtain the best therapeutic effect after PCI. Aspirin together with P2Y12 blockers is an effective combination treatment for CAD patients after PCI, and this effectiveness has been widely recognized [5]. The incidence of hyperuricemia varies with the use of different antiplatelet agents for the treatment of CAD patients. In 2009, the PLATO study published in the New England Journal revealed that serum uric acid (SUA) levels and creatinine levels were significantly high after medication administration [6]. A 2016 study reported that SUA levels were elevated [7] at 30–90 days [7] in patients treated with ticagrelor chronic DAPT, but not in those treated with clopidogrel. However, both studies focused on European and American populations, while only 3.5% of CAD patients in East Asia were treated. Therefore, they did not compare the incidence of hyperuricemia in patients treated with antiplatelet monotherapy nor in patients in East Asia. Therefore, to better understand the incidence of hyperuricemia in CAD patients treated with different antiplatelet therapies in China, more extensive and in-depth studies are warranted.
Materials
Object of Study: In total, 326 patients who were diagnosed with CAD in the Department of Cardiology, the Third Affiliated Hospital of Guizhou Medical University Hospital and The Seventh Affiliated Hospital of Southern Medical University Hospital from March 2021 to December 2021 were selected. After screening these patients, 214 patients received antiplatelet treatment. Based on the antiplatelet regimen received after hospitalization, the patients were divided into four groups. Using the random number table method, 22 patients were assigned to the aspirin group (group A), 24 patients to the clopidogrel group (group C), 79 patients to the aspirin combined with clopidogrel group (group AC), and 89 patients to the aspirin combined with ticagrelor group (group AT). Based on the preoperative blood uric acid levels in the four groups, the blood uric acid levels and the incidence of hyperuricemia in the four groups were observed at 24–48 h and 3–6 months after medication (Figure 1).
Inclusion and Exclusion Criteria: Patients with angina pectoris were enrolled in this CAD study, which includes SA, UA, NSTEMI, and STEMI. The diagnostic criteria are in line with the Primary Diagnosis and Treatment Guidelines for Stable Coronary Heart Disease (2020 edition) and the Emergency Rapid Diagnosis and Treatment Guidelines for Acute Coronary Syndromes (2019 edition). Patients with hemodynamic instability, a malignant tumor, and diseases involving active bleeding or bleeding tendency; those who had used non-steroidal anti-inflammatory drugs, IIb/IIIa antagonists, or oral anticoagulant therapy within the past 4 weeks; and those with a recent surgical history or trauma, febrile illness, acute or chronic inflammatory disease, platelet count < 100,000 /mm3, hemoglobin level < 8.0 g/dL, and severe chronic renal failure (serum creatinine >2.0 mg/dL) were excluded from the study.
Ethical Review: After strict ethical review, this study was approved by the Ethics Committee of the Seventh Affiliated Hospital, Southern Medical University. The study procedure and protocol were fully understood and supported by patients and their families.
Methods
PCI Treatment: Using the Seldinger technique, coronary angiography to more accurately assess the condition and determine the optimal PCI strategy in accordance with the 2018 European Society of Cardiology guidelines for myocardial revascularization. Drug Therapy: Group A was orally administered 100 mg aspirin once daily. Group C was orally administered 75 mg clopidogrel once daily. Group AC were administered 300 mg aspirin and 300 mg clopidogrel once a day as the preoperative load. Group AC received 100 mg aspirin and 75 mg clopidogrel once a day as the postoperative maintenance dose. Group AT received 300 mg aspirin and 180 mg ticagrelor once a day as the preoperative load. Group AT received 100 mg aspirin once daily and 90 mg ticagrelor twice daily as the postoperative maintenance dose.
Observational Indicators
General Data: The groups were compared in terms of sex, age, past medical history (incidence of hypertension, incidence of diabetes, smoking rate, incidence of renal failure, previous PCI, and previous myocardial infarction), type of coronary heart disease (CHD), and type of concurrent medication [angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), beta blockers, nitrates, statins, CCBs, and diuretics].
Biochemical Index Measurement: After 12 h of fasting, blood was collected from the patients in the morning, and count of white blood cells (WBCs) and platelet; levels of hemoglobin, total cholesterol, high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C); and the ejection fraction (EF) value of cardiac color Doppler examination were comprehensively measured. Before study enrollment, blood uric acid levels in all patients were evaluated, which was used as the baseline control. The blood uric acid levels and the incidence of hyperuricemia in the four groups at 24–48 h and 3–6 months after taking the medication were evaluated. According to national regulations, hyperuricemia is diagnosed if blood uric acid levels exceed >416.0 μmol/L (7 mg/dL) in men and >357.0 μmol/L (6 mg/dL) in women.
Statistical Analysis
The SPSS 17.0 software package was used for statistical analysis. Continuous and categorical variables were analyzed using variance analysis and Cuddy test, respectively. Continuous data were expressed as mean ± standard deviation, while categorical data were expressed as percentages. Patients were grouped according to the type of antiplatelet therapy used (aspirin group: A, clopidogrel group: C, aspirin combined with clopidogrel group: AC, and aspirin combined with ticagrelor group: AT). Logistic regression results for hyperuricemia predictors were determined on the basis of load and maintenance doses. By analyzing hyperuricemia predictors, we identified a valid logistic regression model. Logistic regression analysis was performed to investigate the possible causes of hyperuricemia. Using repeated measurements, significant differences in the incidence of hyperuricemia were observed between the groups at different time points. P <0.05 was considered statistically significant.
Of the 326 patients included in this study, only 214 patients completed the treatment after screening. Of these, 22 patients received aspirin alone (group A), 24 patients received clopidogrel alone (group C), 79 patients received aspirin combined with clopidogrel (group AC), and 89 patients received aspirin combined with ticagrelor (group AT).
Comparison of General Information
Table 1 reflects the analysis of the main clinical and demographic characteristics of the patients who completed the treatment. There were 22 cases in group A, including 12 males and 10 females, with an average age of (67.4±11) years; 24 cases in group C, 10 males and 14 females , mean (68.2±12) years; AC group 79 cases, 53 males, 26 females, mean (69.5±11.5) years old; AT group 89 cases, 67 males, 22 females, mean (60.5±13.1) years old ; Among them, the average age of patients in group AC was the highest, and the percentage of women in group C was the highest, and the difference was statistically significant (P<0.05). In terms of taking drugs: ARB drugs and calcium antagonists took the highest proportion in group A; ACEI drugs and nitrate lipid drugs took the highest proportion in group AC, and the difference was statistically significant (P<0.05). There was no significant difference among the four groups in hypertension, hypercholesterolemia, diabetes, smoking history, renal failure, previous PCI, previous MI, clinical diagnosis, taking β-blockers, statins, diuretics, etc. Significant (P>0.05).
Comparison of Major Biochemical Parameters
The results of the main biochemical parameters are presented in Table 2. The HDL-C level was slightly higher in group A than in the other groups (HDL-C = 1.4 ± 0.3 mmol/L, p < 0.01). The uric acid levels at 24–48 h (424.2 ± 137.8 μmol/L, p = 0.035) and at 3–6 months ( 472.6 ± 125.7 μmol/L, p = 0.011) were significantly higher in group A than in the other groups. In addition, the incidence of hyperuricemia in group A was also the highest (77.3%) when the maintenance dose was applied (p < 0.01). The leukocyte level was slightly higher in group AT than in the other groups (WBC = 9.5 ± 4.2*109/L, p = 0.03). No significant differences were observed in other biochemical indices, such as platelet count; levels of hemoglobin, total cholesterol, and LDL-C; and incidence of hyperuricemia, with the loading dose (p > 0.05).
Occurrence of Hyperuricemia May Be Related to Various Factors
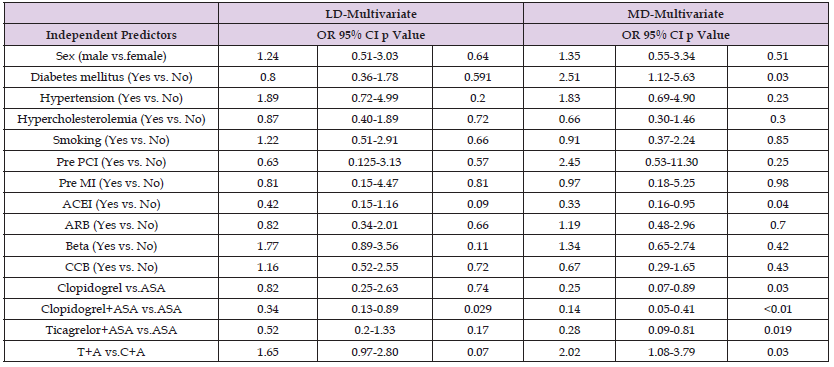
Logistic regression results of hyperuricemia predictors for load and maintenance doses are presented in Table 3. Diabetes mellitus was found to be an independent risk factor for the increased incidence of hyperuricemia at the maintenance dose [odds ratio (OR) = 2.51, 95% confidence interval (CI): 1.12–5.63, p = 0.03]. Among the patients treated with ACEIs, a 33% reduction was observed in the incidence of hyperuricemia at maintenance doses (OR = 0.33, 95% CI: 0.16–1.95, p = 0.4). In the monoclonal antibody-treated groups, a 25% reduction in hyperuricemia was observed at the maintenance dose in group C compared with group A (OR = 0.25, 95% CI: 0.07–0.89, p = 0.03). In the dual antibody-treated groups, the incidence of hyperuricemia at the maintenance dose was significantly higher in group AT than in group AC (OR = 2.02, 95% CI: 1.08–3.79, p = 0.03). By contrast, the incidence of hyperuricemia at the maintenance dose was 14% lower in group AC than in group A (OR = 0.25, 95% CI: 0.05–0.41, p < 0.01). Compared with group A, the incidence of hyperuricemia at the maintenance dose was reduced by 28% in group AT (OR = 0.28, 95% CI: 0.09–0.81, p = 0.019).
Table 3: Logistic regression results for predictors of hyperuricemia on the basis of loading and maintenance doses.
Repeated Measurements of Blood Uric Acid Levels in Cad Patients
After antiplatelet drugs were administered, blood uric acid levels of CAD patients exhibited significant differences at different times. As observed in Figure 2, the uric acid level in group A was significantly higher than that in the other groups at both 24–48h and 3–6 months. The uric acid level of patients treated with the monoclonal antibody was significantly higher than that of those treated with dual antibodies at 24–48 h or at 3–6 months. The uric acid levels in groups A, AC, and AT at 24–48 h had increased at 3–6 months, indicating that the incidence of drug-induced hyperuricemia also increases with time.
Many advancements have been made in CAD therapy over the past few years, and these advancements have improved patient outcomes. The multifactorial pathogenesis of atherosclerotic thrombotic disease is not yet completely understood. Understanding this pathogenesis would help identify the risk factors involved and reduce mortality. Hyperuricemia is a common risk factor having serious consequences. Internationally, hyperuricemia is diagnosed as a blood uric acid level of >420 μmol/L (7 mg/dL) in men and >357 μmol/L (6 mg/ dL) in women.
Hyperuricemia and Cardiovascular Events
A recent study exhibited a significant increase in the hyperuricemia rate among people with high blood pressure; hyperuricemia is not only an independent risk factor but also increases the risk [8- 10] for patients with high blood pressure. The findings suggest that hyperuricemia contributes to the development of coronary atherosclerotic heart disease as well as chronic heart failure; this finding has been well established. A meta-analysis [11] revealed that a 1 mg/ dL increase in hyperuricemia was associated with a 12% increase in mortality from CHD. In patients with chronic heart failure, blood uric acid levels were inversely correlated with the cardiac EF and were unaffected by kidney function and diuretic drugs [12]. According to the latest findings [13], a significant positive correlation exists between blood uric acid levels and mortality in moderate-to-severe heart failure, a finding that offers an accurate diagnostic method for assessing the prognosis in this patient group. Moreover, hyperuricemia is an independent risk factor [14]. From a pathogenicity perspective, uric acid has been reported to increase oxidative stress, endothelial dysfunction, and smooth muscle cell proliferation [15,16].
Changes in Aspirin and Blood Uric Acid Levels
Drug-induced hyperuricemia has become a new and increasingly common clinical problem. Being a classic antiplatelet aggregation drug, aspirin is commonly used in CHD patients and after coronary artery stenting. Aspirin has played a crucial role in reducing morbidity and mortality due to cardiovascular events. However, at the same time, aspirin is a drug that causes elevation of uric acid levels. In a recent large prospective case-crossover study [16], low-dose aspirin use (≤325 mg/day) was associated with a higher risk of recurrent gout attacks, and this risk increased as the dose decreased. When aspirin is used in hypoalbuminemia patients or in combination with diuretics, hypoalbuminemia and diuretics enhance the uric acid retention effect of aspirin [17], further increasing the blood uric acid levels. In this study, uric acid levels in the aspirin group (group A) were significantly higher than those in the other groups at both 24–48h or after 3–6 months of retesting. This result is consistent with those of previous studies, and the possible underlying mechanism is that aspirin can inhibit prostaglandin production, thereby leading to the contraction of renal arterioles. This thus reduces renal blood flow, which in turn reduces uric acid excretion and ultimately leads to hyperuricemia [18].
Clopidogrel and Changes in Blood Uric Acid Levels
As a P2Y12 receptor antagonist, Wang Yue [19] found that clopidogrel has no significant effect on blood uric acid levels when used alone. In an experiment [20], we found that the incidence of hyperuricemia was significantly reduced in patients receiving clopidogrel. This result provides a strong support for hyperuricemia treatment in CAD patients and offers strong evidence for the efficacy of the clopidogrel and aspirin combination in UA treatment. Thus, the results provide a critical reference for improving the health status of patients. Thus, clopidogrel combined with aspirin is effective. Although inconsistent with previous study results, this may take consider the genetic polymorphism of clopidogrel, which varies in efficacy among different ethnic groups, the occurrence of hyperuricemia, and the incidence of cardiovascular events.
Ticagrelor and Changes in Blood Uric Acid Levels
Recent evidence revealed that clopidogrel-resistant and [21,22] HRPR patients have the worst [21,22] outcomes, a problem that was only partially overcome with using new, more effective ADP antagonists such as ticagrelor. Ticagrelor is a specific P2Y12 receptor inhibitor that is active [23] without being metabolized, which allows it to inhibit platelet production better than clopidogrel, thus greatly reducing the probability [24]. However, one side effect of ticagrelor is that it increases uric acid levels. In one study [25], ticagrelor significantly increased [25] uric acid levels compared with clopidogrel. During the planned 12-week follow-up period, patients treated ticagrelor for gout attacks experienced joint-related adverse events. In a recent study, when healthy male volunteers took 90 mg/bid ticagrelor, their blood uric acid levels were 1 day higher (4%–6%) than those in the placebo group. On day 5, the data showed a 4%–10% decrease, but then the levels quickly recovered to the normal levels [26]. This result suggests that ticagrelor has the potential to contribute to hyperuricemia development.
However, in this experiment, the incidence of maintenance dose-induced hyperuricemia was significantly increased in group AT compared with group AC, which proves that ticagrelor causes a certain increase in the incidence of hyperuricemia; this result is consistent with those of many studies. According to the latest research, the presence of ticagrelor and its metabolites may block adenosine intake by cells, resulting in increased extracellular and intravascular levels of adenosine, which further [27] activates uric acid production. Extracellular and blood levels of adenosine lead to increased uric acid synthesis in tissues with high xanthine oxidase levels. Ticagrelor can significantly improve blood perfusion in CAD patients because of the elevated adenosine levels. This improvement can significantly enhance coronary blood perfusion, thus giving ticagrelor a distinct advantage in terms of long-term benefits.
Furthermore, the incidence of hyperuricemia at the maintenance dose was lower in group AT than in group A in this study. Indirect evidence that aspirin does increase uric acid levels is consistent with the findings of previous studies. [28,29] To sum up, in clinical practice, the selection of antiplatelet drugs varies with differences in patient individuation. However, the effect of different combinations of antiplatelet drugs on blood uric acid levels in CAD patients is easily overlooked. Hyperuricemia, as a metabolic disease, in addition to causing gout attacks, is also an independent risk factor for cardiovascular disease. It can also accelerate the progression of coronary atherosclerosis. Our study confirmed that patients on clopidogrel had a reduced incidence of hyperuricemia. Patients treated with the combination of aspirin and ticagrelor had a considerably greater risk of hyperuricemia than those treated with clopidogrel. To ensure the health of patients after CAD, attention must be focused not only on the reduction of blood lipids, blood sugar, and blood pressure but also on the control of uric acid levels, and therefore, oral therapy with the aspirin and ticagrelor combination is essential. After taking drugs, blood uric acid levels of the patients should be tested periodically to timely adjust the treatment plan and take early intervention measures. This would thus help in the better and quicker restoration of health of CHD patients and realize accurate treatment.
When antiplatelet agents were used alone for the treatment of CAD patients, the incidence of hyperuricemia was significantly lower in the clopidogrel group than in the aspirin group. When the patients were treated with dual antiplatelet agents, the incidence of hyperuricemia in the aspirin+clopidogrel group was significantly lower than that in the aspirin+ticagrelor group. Therefore, the antiplatelet regimen for CAD patients should be selected on the basis of their basal uric acid values. Finally, when treating patients with high uric acid levels by using aspirin and a dual antiplatelet regimen, physicians should take uric acid management seriously. They should recommend patients to test their uric acid levels regularly after 3 months of treatment so that the treatment regimen can be adjusted accordingly, and early intervention measures can be taken for hyperuricemia if required.
This paper was supported by the Guangdong Provincial Department of Education (no.2021KTSCXO18), the Project of Guangdong Science and Technology Department (No. 10202H20200035) .
The authors declare no conflict of interest.


