Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Lydia Fructuoso González*, Noemí Manresa Ramón, María Dolores Najera Perez and Paula Torrano Belmonte
Received: June 29, 2023; Published: July 06, 2023
*Corresponding author: Lydia Fructuoso-González, Hospital Universitario José Morales Meseguer, Hospital Pharmacy Service, Murcia, Spain
DOI: 10.26717/BJSTR.2023.51.008097
We report the case of a 72-year-old man with chronic myelomonocytic leukemia (CMML) who underwent allogeneic hematopoietic cell transplantation (HCT). In the setting of graft-versus-host disease (GVHD) stage 3, involving gastrointestinal tract, the patient received treatment with isavuconazole, which could subsequently lead to the emergence of nail alterations that have not been previously documented with this medication.
Keywords: Hematopoietic Cell Transplantation; Graft-Versus-Host Disease; Antifungal; Isavuconazole; Nail Changes
Abbreviations: CMML: Chronic Myelomonocytic Leukemia; HCT: Hematopoietic Cell Transplantation; GVHD: Graft-Versus-Host Disease
Allogeneic HCT is the only potentially curative option for individuals diagnosed with CMML. However, this procedure is accompanied by GVHD and other factors contributing to non-relapse mortality. Despite transplantation leading to long-term disease control/cure in 20 to 50 percent of cases, the eligibility criteria for allogeneic HCT are restricted to less than 20 percent of patients, with less than 5 percent actually undergoing the procedure due to a median age exceeding 70 years at presentation [1]. Antifungal are frequently employed for prolonged periods of time (ranging from weeks to months), especially in individuals afflicted with hematologic malignancies or those who have undergone hematopoietic stem cell transplantation. Certain adverse reactions have been associated with the utilization of triazoles, encompassing hepatotoxicity, peripheral neuropathy, skin rashes, visual hallucinations, and heart failure [2,3]. Voriconazole, in particular, has been linked to various adverse effects, including visual, hepatic, dermatologic, and neurotoxic manifestations [4]. Multiple studies have indicated that isavuconazole exhibits a high level of tolerability and a favorable safety profile. [2,5,6] The SECURE trial demonstrated that patients treated with isavuconazole experienced lower incidence rates of hepatobiliary disorders (9%), eye disorders (15%), and skin disorders (33%) compared to those treated with voriconazole [2].
A 72-year-old man underwent an allogeneic peripheral hematopoietic stem cell transplant with reduced intensity conditioning (RIC) regimen from a familial haploidentical donor, ABO minor incompatible in May 2020. The patient was diagnosed with CMML in a state of refractory pancytopenia after 12 cycles of 5-azacitidine. In the context of chronic grade 1 and acute grade 3 cutaneous and digestive GVHD, in February 2023, the patient started treatment with topical and systemic corticosteroids: prednisone (1mg/kg/day), as well as extracorporeal photopheresis. Two months ago, in April 2023, the patient initiated prophylactic antifungal treatment with isavuconazole. In June 2023, he presented with nail alterations, as shown in (Figure 1), and visited the outpatient pharmacy service to collect his medication. The patient reports that this side effect does not bother him. Unfortunately, neither the hematologic nor the dermatology service has been able to determine the cause of the nail alteration.
Figure 1 Nail changes allogeneic hematopoietic cell transplantation in treatment with isavuconazole.
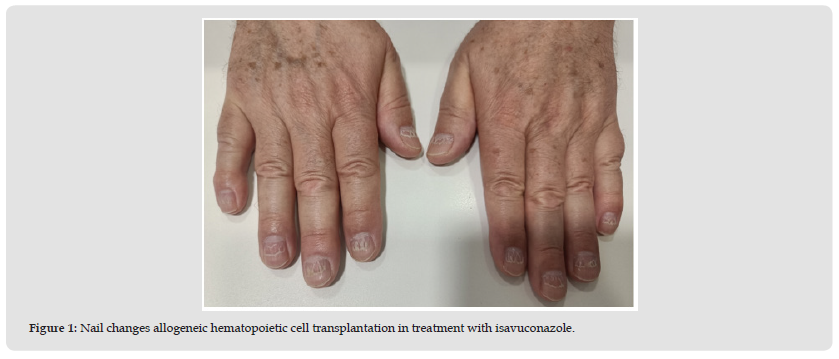
Nail changes are not commonly reported side effects of voriconazole [7], and it has not been reported as a side effect of isavuconazole before. Unfortunately, we did not specifically define further the effect on nails, and additional study is needed to elucidate the relationship between isavuconazole and this effect. The patient was also subjected to high-dose prednisone therapy (1mg/kg/day) three months preceding the onset of the nail alterations, while concurrently receiving, extracorporeal photopheresis for the management of gastrointestinal graft-versus-host disease. Subsequently, a direct association with the administration of isavuconazole cannot be established; however, it remains one of the potential causative factors.
The authors have not declared a specific grant for this research from any funding agency in the public, commercial, or not-for-profit sectors.


