Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Nathalia Fonseca Boiani*, Marcelo Miyada Redígolo, Wilson Aparecido Calvo, Flávio Kiyoshi Tominaga and Sueli Ivone Borrely
Received: June 28, 2023; Published: July 05, 2023
*Corresponding author: Nathalia Fonseca Boiani, Instituto de Pesquisas Energéticas e Nucleares, Radiation Technology Center – IPEN-CNEN/SP, São Paulo, SP, Brazil
DOI: 10.26717/BJSTR.2023.51.008096
The wide use of pharmaceuticals and water scarcity are associated to increasing levels of pharmaceutical compounds discharged into water and wastewater worldwide, affecting relevant ecological services, including biodegradation. However, water pollution has also encouraged studies applying advanced oxidative processes (AOP) in organic pollutant degradation. Among AOPs, ionizing radiation has been proven an effective technology for organic compound removal from waters and wastewater. The objective of this study was to assess Electron Beam (EB) irradiation in the degradation of caffeine and fluoxetine and their binary mixture in pure aqueous solutions. The degradation of these pharmaceuticals was evaluated by GC/MS analyses. The degradation dose response was higher for the caffeine and fluoxetine mixture (1:1) at 2.5 kGy. This dose led to decreased toxicity towards Daphnia similis for both the fluoxetine + caffeine mixture and the isolated fluoxetine solution, but not for the isolated caffeine. On the other hand, Vibrio fischeri exposure for 15 min indicated toxicity removal for the entire pharmaceuticals sample set and radiation dose. Fluoxetine was the most toxic pharmaceutical, followed by the binary mixture. Thus, we suggest ecopharmacovigilance, where attention should be paid to the increasing amount of pharmaceuticals, caffeine and fluoxetine detected in water
Keywords: Caffeine; Fluoxettine; Mixture of Pharmaceuticals
Abbreviations: AOP: Advanced Oxidative Processes; EB: Electron Beam; PPCPs: Pharmaceuticals and Personal Care Products; WWTP: Wastewater Treatment Plants
The worldwide overuse of pharmaceuticals and personal care products (PPCPs) has introduced these compounds in exorbitant amounts into aquatic systems. Pharmaceuticals in particular have been highlighted among other water contaminants not only due to their significant consumption and continuous discharges into aquatic environments, but also to their manufacturing processes. Wastewater Treatment Plants (WWTP) are one of the main PPCP sources to aquatic systems, as they receive domestic and hospital sewage, as well as pharmaceutical effluents, from production sectors, all of which may contains extremely high pharmaceutical levels [1-3]. Antimicrobial resistance development is directly associated to the overuse of antibiotics [4], and a systematic review on the compounds of greatest concern in this regard emphasized seven antibiotics among 45 main residues associated to environmental risks and bacterial resistance [5]. The occurrence and fate of antibiotics, their resistant genes, and their wide presence in WWTPs [2], as well as WWTP limitations regarding pharmaceutical degradation must be considered and technical innovations are required [6-8].
One of the main techniques applied to organic pollutant degradation comprises advanced oxidative processes (AOP). Ionizing radiation is an effective AOP in this regard, applied with gamma rays and electron beam (EB) accelerators. Both techniques are based on reactive species obtained during water radiolysis [9], which react with organic compounds in water. Several studies have been carried out in this regard, for example applying EB to antibiotic degradation [10,11] and gamma radiation for fluoroquinolone decomposition [12]. Due to their limited biodegradability, fluoxetine and caffeine comprise two PPCPs increasingly present in different environmental matrices, resulting in risks to aquatic systems and human health [3]. In Brazil, for example, among hundreds of active pharmaceutical ingredients, caffeine was pointed out as a ubiquitous contaminant in rivers and reservoirs in the state of São Paulo, while fluoxetine, a selective serotonin re-uptake inhibitor, has been indicated as a potential environmental risk to Brazilian water quality [13]. Most data concerning pharmaceutical degradation is, however, based on isolated products assessed in pure water solutions, indicating the need for studies concerning pharmaceutical mixtures and their metabolites. Therefore, the aim of the present study was to evaluate the efficiency of EB irradiation in the degradation and toxicity reduction of fluoxetine, caffeine, and their binary mixture in aqueous solutions, in order to assess the potential of this technique as an option for wastewater treatment.
Reagents
Caffeine (CFN) [C8H10N4O2; MM = 194.19 g mol−1; 1,3,7-Trimethylpurine-2,6-dione; CAS 58-08-2], was obtained from Sigma-Aldrich® (purity ˂ 98%); Fluoxetine hydrochloride (FLX) [C17H18F3NO. HCl; MM = 309.33 g mol−1, mehyl [(3S)-3-phenyl-3-[4-(trifluoromethyl) phenoxy] propyl] amine]; CAS 54910-89-3] were purchased from Divis Pharmaceuticals Pvt. Ltd. (98.8% purity).An initial caffeine concentration of 100 mg L-1 and fluoxetine of 10.0 mg L−1 for the individual degradation studies was used. For the mixture experiments, the initial solution was prepared at 50 mg L−1 of caffeine and 10 mg L-1 of fluoxetine. A binary mixture of both pharmaceuticals was prepared by mixing each aqueous solution at a 1:1 ratio.
Sample EB Irradiation
All samples were irradiated in batches employing a Dynamitron® Electron Beam Accelerator set at 1.4 MeV for all experiments. The conveyor speed was set at 6.72 m min-1 for samples passing under the electron beam and the electric current varied according to the required irradiation dose. The samples were placed in rectangular glass 246 mL-recipients (Pyrex®) to ensure a suitable beam penetration of 4 mm and irradiated at 1.0, 2.5 and 5.0 kGy. Doses were confirmed using a Perspex Harwell Red Batch KZ- 4034 dosimeter, with less than 5% variation.
Analytical Techniques
The caffeine/fluoxetine mixture was assessed employing a Shimadzu QP2020 model gas chromatography mass spectrometer (GC/MS). The GC was set to 70°C for 1 min, increasing to 300 °C at 8 °C min-1 and held for 1 min. The injector was set at 250 °C. A 1 mL min-1 flow rate was applied, with a 10 split. The MS interface was set at 280 °C, the source as 250 °C and the m/z range from 35 to 350.
Toxicity Assays
Acute toxicity assays with the microcrustacean Daphnia similis were carried out based on the ABNT/NBR 12713/2016 standard [14], using natural water. All assays were performed in duplicate and consisted of exposing juvenile individuals to samples for 48 h (20 juveniles per sample dilution) to the samples in a BOD incubator. The observed effect was immobility/mortality. Toxic effects were assessed by median effect concentration values affecting 50% of the exposed organisms (EC50%), calculated by the Trimmed Spearman-Karber method [15]. Marine bacteria Vibrio fischeri luminescence was assessed in an acute toxicity screening assay carried out according to the ABNT/NBR 15411-3/2012 standard employing an M- 500Microtox System [16]. The statistical analysis used to determine EC50% values was based on the gamma value (relation between lost and remaining luminescence for each sample dilution). Data from I0 and I15 were analyzed by linear regressions. The assays were performed in duplicate with four different pharmaceutical concentrations. Toxicity removal (%) from the samples after EBI treatment was calculated according to Equation 1.

Where: TUo = Toxicity Units before irradiation and TUirrad = Toxicity Units after irradiation, and EC 50% = median effective concentration.
The significance of differences between the mean control (non-irradiated) sample values and experimental (irradiated) sample values were assessed by an analysis of variance (ANOVA) at a 5% significance threshold.
Chromatographic analyses (GC/MS) were carried out to quantify and confirm EB degradation efficiency by comparing peak compound areas. The caffeine and fluoxetine was analyzed by GC/MS and presented in Figure 1. Therefore, peak areas were calculated at 2.5 kGy, where caffeine and fluoxetine display 99% and 100% degradation, respectively. It is important to note that, when analyzed as separate compounds by GC/MS, caffeine exhibited only a 43% degradation, while fluoxetine was degraded by 98%. This impressive degradation efficiency gain for caffeine may be due to a decrease in competing species promoted by the presence of fluoxetine. Acute toxicity assay data are exhibited in Figures 2 & 3. The toxicity values are expressed as EC50%, an inversely proportional number, as a lower amount of a certain compound is required to cause an effect in 50% of exposed organisms. The findings evidenced D. similis as more sensitive to the tested pharmaceuticals compared to V. fischeri. Fluoxetine was significantly more toxic to exposed organisms than caffeine, both isolated and in the binary mixture. The decreasing EC50% values observed following caffeine irradiation indicate a more toxic effect in D. similis and less toxic effect in V. fischeri, possibly due to radiation-induced by-products.
Figure 1 Fluoxetine(A) and caffeine(B) chromatograms before and after EB irradiation (1.0 kGy). Initial concentration: [CFN]0 = 50 mg L−1, [FLX]0 = 10 mg L−1.
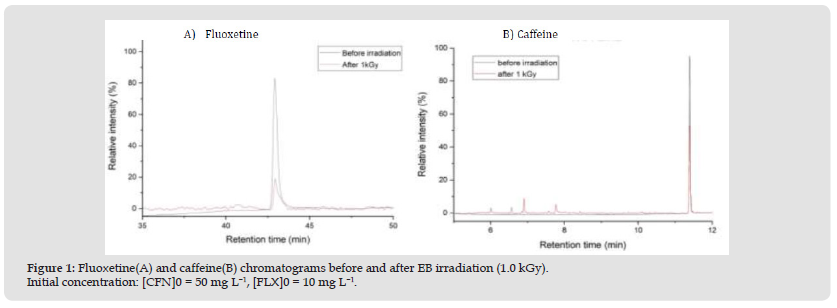
Figure 2Daphnia similis EC50% values following 48 h of exposure for caffeine, fluoxetine and their mixture (fluoxetine + caffeine), versus EB irradiation dose (Tukey’s test, p < 0.05).
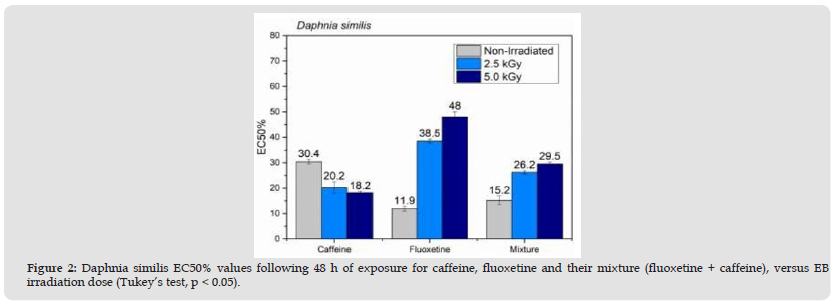
Figure 3 3: Vibrio fischeri EC50% values following 48 h of exposure for caffeine, fluoxetine and their mixture (fluoxetine + caffeine), versus EB irradiation dose (Tukey’s test,p < 0.05).
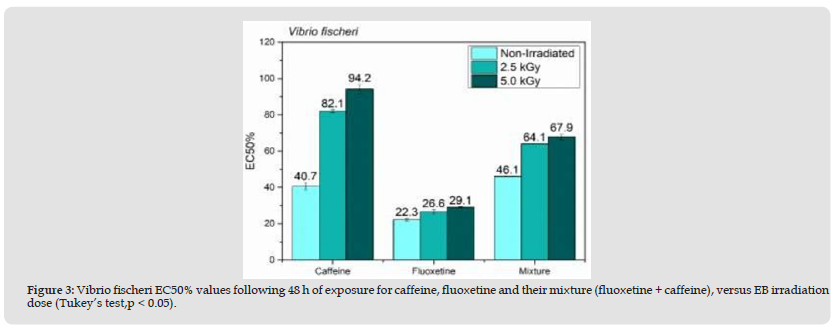
The EB irradiation was efficient in removing acute effects of fluoxetine and the caffeine fluoxetine mixture effects, following the 2.5 and 5.0 kGy doses towards V. fischeri, also observed for caffeine. Caffeine levels in aquatic ecosystems and adverse aquatic organism effects have been reported in multiple aquatic studies [17], the latter due to the fact that only a small fraction of the total consumed caffeine is excreted in its original form [18]. Maximum concentrations of caffeine in raw wastewater (3.6 mg L-1), treated wastewater (55.5 mg L-1), river (19.3 mg L-1), drinking water (3.4 mg L-1), groundwater (0.638 mg L-1), lake (174 mg L-1), catchment (44 6 mg. L-1), reservoir (4.9 mg L-1), and rainwater samples (5.40 mg L-1) have been reported [19]. A 2.5k kGy was enough dose to reduce fluoxetine and the binary mixture effects for both exposed organisms. The same pattern was noted for caffeine in V. fischeri exposed the mixture, but not to pure caffeine. Other studies indicate fluoxetine degradation > 90% at 0.5 kGy [20] and caffeine degradation by gamma radiation at 3.0kGy [21]. The authors also combined ozone and gamma irradiation, and hydrogen peroxide in order to reduce the applied radiation dose. Combined ionizing radiation is, thus, a potential option for wastewater containing pharmaceutical compounds [22].
Increasing fluoxetine concentration in effluents is an important issue due to their relatively low biodegradability, and wastewater cleaning technologies are necessary for the reduction of environmental effects due to pharmaceuticals discharges in water matrices worldwide. Electron Beam irradiation of caffeine and fluoxetine in aqueous solutions was effective for their decomposition and toxicity removal, especially when binary mixture of both were irradiated. Regarding radiation doses, 0.5 kGy and 2.5 kGy were suitable for degradation of fluoxetine in pure water solution, and binary mixture (fluoxetine + caffeine), respectively. Interactions between pollutants and pharmaceuticals have to be taken into account. Fluoxetine was significantly more toxic to exposed organisms than caffeine. Upgrading wastewater treatment plant with advanced technology is recommended as part of actions to reduce pharmaceuticals in aquatic environments.
The Authors would like to thank the Comissão Nacional de Energia Nuclear (CNEN), Instituto de Pesquisas Energéticas e Nucleares (IPEN) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).


