Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Kajari Das1, Ch Surya Kanta Mishra1, Steetprangya Champatiray1, Madhusmita Jena1, Soumya Prasad Panda2 and Pragyan Roy1*
Received: June 06, 2023; Published: June 23, 2023
*Corresponding author: Pragyan Roy, Department of Biotechnology, College of Basic Science and Humanities, OUAT, Bhubaneswar 751003, India
DOI: 10.26717/BJSTR.2023.51.006053
Secondary metabolites in plants like phenols, terpenoids, flavonoids, polyphenols, and alkaloids are used as stabilizing and reducing agents for the synthesis of silver nanoparticles. In the current study tectona grandis teak leaf extract was used for the reduction of silver nitrate to silver nanoparticles. Synthesis of silver nanoparticles was confirmed by colour change from pale red to brown color solution and further characterized by UV-Vis Spectroscopy, DLS. In the past decade, several researchers have reported on the toxic mechanism of silver nanoparticles on several plant species. The present study demonstrates the phytostimulatory effect of green synthesized silver nanoparticles (AgNPs) on Vigna radiata seed germination and growth. All the experimental concentrations of AgNPs (5ppm, 10ppm, and 20ppm) induced an increase in biomass and length of the seedling. A steady increase in the chlorophyll content was also seen in the treated plants as compared to the control. The highest germination percentage of 95% and vigor index of 760 was recorded for plants treated with 20ppm AgNPs. The maximum increase in root and shoot length was 21.25% and 22.3% respectively. CAT activity was highest with a 1.87 folds increase, SOD 1.6 folds and Peroxidase 1.64-fold increase when compared to control. It appears that these antioxidant enzymes were capable of scavenging ROS responsible for oxidative stress in crops. Absorption of silver nanoparticles by seedlings roots and shoots was detected by ICP-AES. At an optimum concentration increase in plant, biomass was observed. Here we report that 20ppm of AgNP has a phytostimulatory effect on the Vigna radiata seedling.
Keywords: Nanoparticles; Teak Leaves; Phytostimulator; Vigna radiata Seeds
Nanobiotechnology is an upcoming field of research where plants and plant extracts are used for the synthesis of nanoparticles. In India, Ayurveda emphasizes the use of medicinal plants for the treatment of diseases. This erudition is again used in the green synthesis of nanoparticles. Chemical and physical methods of synthesis of nanoparticles are not only very expensive but also leave many hazardous chemicals as remnants that are non-biodegradable. Diverse methods have been used for the synthesis of silver NPs including radiation (Dimitrijevic, et al. [1]), chemical (Sun, et al. [2]), electrochemical (Yin, et al. [3]) photochemical methods (Callegari, et al. [4]) and Langmuir-Blodgett (Swami, et al. [5,6]) and biological techniques (Naik, et al. [7]). As a biogenic method for the synthesis of silver nanoparticles are safer and greener and can be easily upscaled. Owing to their eclectic application in diverse fields like water purification (Lin, et al. [8]), pharmaceuticals (Kumar, et al. [9]), medicinal devices (He, et al. [10]) as probes in detection of DNA (Thompson, et al. [11]), and food adulterants (Ping, et al. 12]). Recently different parts of plants like the leaf of Mimusops ellengii (Prakash, et al. [13]) and Azadiracta indica (Roy, et al. [14]), the bark of Cinnamon zeylanicum (Sathishkumar et al. [15]), seeds of Jatropha curcas (Bar, et al. [16]) and roots of Morinda citrifolia (Suman, et al. [17]) are used for the synthesis of silver nanoparticles. Plants contain many secondary metabolites and carbohydrates, proteins, fats, nucleic acids, and pigments which act as reducing and capping agents to produce nanoparticles from metal salts.
Tectona grandis, popularly known as teak or Sagun is used for making furniture, cabinets, and musical instruments. It is a deciduous tree that originated in India belonging to the family Lamiaceae. The leaves are very large, shiny, and hairy below. In the ayurvedic system both flowers and seeds are considered diuretics, the wood acts as a laxative and sedative for uterus, effective against piles and dysentery. Bark is also used in the treatment of diabetes and bronchitis. Its leaves, bark, and wood show antioxidant properties, with wood showing a maximum of 98.6%inhibition against DPPH (Krishna, Jayakumaran [18]). Diallo, et al. [19] have reported the anti-hemolytic anemia activity of teak, which can be used in the treatment of anemia. An antibacterial compound Juglone is present in its bark which has shown antibacterial against methicillin-resistant Staphylococcus aureus (MRSA) and Listeria monocytogenes (Neamatallah, et al. [20]). Due to the indiscriminate and manifold use of silver nanoparticles, it is very essential to understand the toxicity pathway of nanoparticles. Nanoparticles enter the soil microsphere by agricultural application, rainwater, surface run-offs, or other pathways and are retained in the soil for a long time due to their low migration. As primary producers plants obtain all kinds of essential minerals and nutrients from the soil. Thus nanoparticles are also absorbed from the soil and plants become a potential pathway for the transport of nanoparticles. Like the food pyramid, the concentration of nanoparticles goes on increasing at every trophic level. So it is very important to study the phytotoxicity of nanoparticles on plants, especially with respect to certain physical parameters.
In the present study, Tectona grandis leaf extract was utilized for the reduction of silver nitrate to silver nanoparticles, and characterization of the synthesized nanoparticles was carried out by UV-visible spectroscopy and DLS. Antimicrobial activity of the green synthesized nanoparticles was observed against typical human pathogenic microorganisms Escherichia coli and Staphylococcus aureus. The minimum inhibitory concentration of silver nanoparticles against these microorganisms was determined. Besides, the main physiological indices of mung bean plants like germination percentage, root elongation, biomass, chlorophyll and protein content were studied. Phytotoxicity of silver nanoparticles on mung bean was monitored with respect to the release of reactive oxygen species.
Experimental Design
Teak leaves were used for synthesis of silver nanoparticles and the synthesized nanoparticles were characterized by DLS. To each 5 ppm, 10 ppm, and 20 ppm AgNPs, 10ml MS medium was added, vortexed gently, and immediately cooled in ice. After solidification of medium Vigna radiata seeds were pushed gently on the medium. Seeds were allowed to grow in a photoperiod of 12h light and 12h darkness. The seeds were observed for root length, shoot length, appearance of leaf size, colour and chlorophyll content for fifteen days. All the samples were processed in triplicates.
Green Synthesis of Silver Nanoparticles
Collection and Identification of Tectona Grandis Leaves: Tectona grandis or teak leaves were collected from the College of Basic Sciences and Humanities Campus, Bhubaneswar Odisha. Fresh and green leaves were collected in the month of March 2019 (Figure 1).
Preparation of Tectona Grandis Leaves Extract: The fresh teak leaves were collected and washed thoroughly in running water to remove dirt. Then it was cleaned with double distilled water and shade dried for 10 days. Leaves were crushed into powder form in a mixer and stored in airtight container. Twenty grams of powder teak leaves were added to a beaker containing 100 ml of distilled deionized water. The mixture was heated on a hot plate for 20min at 60°C, till the colour of the extract became wine red. The mixture was allowed to cool and filtered through Whatman no.1 filter paper. Freshly prepared aqueous extract of teak was maintained in an airtight container at 4°C for further use.
Preparation of 1 mM of Silver Nitrate Solution: To seventeen milligrams (17 mg) of silver nitrate (Merck laboratories (P) Ltd. 99.9% AgNO3, MW = 169.87 g/mol) 100 ml of double distilled water was added in 500ml Erlenmeyer flask. The silver nitrate was slowly dissolved by gently mixing the deionized water. The prepared silver nitrate solution was stored at 4°C in amber colored bottle.
Synthesis of Silver Nanoparticles: To 90 ml of 1 mM of silver nitrate solution 10 ml of aqueous extract of teak, leaves was added. Leaf extract has many stabilizing and capping agents that bring the reduction of Ag+ ions to Ag nanoparticles. The reaction was allowed to take place at room temperature undisturbed, for 48hrs. After the incubation period, the mixture containing the product was centrifuged at 7000rpm for 10 minutes. This step was repeated three times for proper dispersion of nanoparticles.
Characterization of Silver Nanoparticles: UV-vis spectrophotometer: After 30 minutes, a slight colour change was observed from the addition of silver nanoparticles to the leaf extract. A distinct change in colour from wine red colour to amber colour was seen after 24hrs. Indication of bio reduction of Ag+ ions in the teak extract was evidenced by monitoring the absorption spectra in a UV-vis spectrophotometer (T70/T80 series UV/Vis spectrophotometer PG Instrument Ltd., England). The spectrum of the reaction mixture was at a resolution of 1nm in the range of 800 to 200nm.
Dynamic Light Scattering: This method was used to predict the size of Ag nanoparticles and their distribution in an aqueous medium. Data on particle size distribution was extracted in Zetasizer (Malvern instruments).
Preparation of MS Medium
Murashige and Skoog’s semi-solid (MS) medium (Murashige and Skoog’s medium) at a strength of 1/20 was prepared as per the manufacture’s instruction. To the nutrient medium, 3% sucrose was added as the carbon source, and 0.8% agar was added for solidification. The pH of the medium was set to 5.8.
Addition of Silver NPs (Nanoparticles) into the MS Medium
Silver nanoparticles were filtered by passing through a sterilized Millipore filtration system having a pore size of 0.3 µm attached to a clinical syringe. Then 5 ppm, 10 ppm, and 20 ppm were added to the culture test tubes and 10 ml of medium was added to each test tube. For uniform dispersion of nanoparticles the tubes were vortexed for five minutes and immediately stored in an ice chamber so that the medium polymerizes faster. MS medium without nanoparticles was considered as the control.
Toxicity Studies
Effect on Seed Germination: Vigna radiata seeds were surface sterilized in a 5% solution of sodium hypochlorite and then washed thoroughly in water. The seeds were soaked in sterile distilled water for 12hrs and then kept in dark for 12hrs. Then 3 concentrations of Ag nanoparticles (5, 10, 20 ppm) were added to a different test tube containing 10ml of MS medium. The following equation was used to calculate germination percentage (%G).
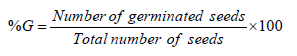
Effect on Root and Shoot Length: After incubation on agar, the seedlings were observed for growth in root length, shoot length and changes in the direction shape of terminal and secondary roots as compared to the control. The experiment was conducted in triplicate in which each concentration of AgNPs had three seedlings. After incubation, the seedlings were collected and the root length, shoot length, number of leaves, and wet weight were measured. Vigor index was calculated based on Vashisth and Nagarajan equation (Vashisth,Nagarajan [21]):

Biomass Assay and Ag Concentration in Roots and Shoots: After fifteen days the roots and shoots were washed thoroughly to determination of biomass. Initially fresh weight of roots and shoots was measured after 3 days, 5 days, 7 days, 11 days, and 15 days. Then they were dried in an oven at 70°C for 24hrs. Dried shoots and root samples were dried on a hotplate with 1ml of HCl and 1ml of Hydrogen peroxide. Samples were reconstituted in 1% HNO3 and Milli-Q water. After complete drying, the dry weight was recorded. Blanks were made with the same solvents and chemicals used in digestion. Then the amount of silver that was retained in the root was determined by ICP-AES (Inductively coupled Plasma- atomic Emission Spectroscopy) in Model Ultima, Jobin Yvon Company, France after nitric acid digestion of dry roots and shots.
Protein Estimation: The quantification of total protein was done by the Folin Lowry method (Lowry et al., 1951). First shoot samples were homogenized in ice using 50 mM sodium phosphate buffer (pH 7.8, 2% (w/v) 1 mM EDTA and polyvinylpyrrolidone (PVP) and then centrifuged at 12,000 × g for 10 min at 4°C. Three solutions; A (alkaline solution), B (copper fsulfate-containing solution), and C (sodium tartrate solution) were prepared. Solutions A, B, and C were mixed in the ratio 100:1:1 to make the Lowry solution. The dilution ratio of Folin Ciocalteau’s Phenol reagent is 1:1 to make 1N Folin reagent. BSA standard stalk solution was prepared before the experiment. To 0.5ml protein lysate 0.7ml of Lowry solution was added and incubated in dark for 20 minutes. After incubation 0.1ml of Folin reagent was added and again incubated at room temperature for 30 minutes. After 30 minutes of incubation at room temperature absorbance was measured at 750nm in Spectrophotometer. Aqueous BSA was taken as standard and protein content was expressed as mg/g wet weight of tissue.
Analysis of Oxidative Stress: Total protein estimation following the Folin Lowry method (1951) was done with BSA as standard. Superoxide dismutase (SOD), Peroxidase (POD), and Catalase (CAT) activities were determined using the leaf extract. Homogenization of 100 mg of leaves was done in 1 ml of ice cold 100 mM phosphate buffer (pH 7) having 1 mM PMSF (phenylmethylsulfonyl fluoride), 0.1mM EDTA (ethylenediaminetetraacetic acid), 2% PVP (polyvinylpyrrolidone) and 0.2% TritonX-100. After centrifugation at 12000 g at 4°C for 20min the supernatant was used as the enzyme extract. POD activity of the leaf extract was determined by the Kar and Mishra (Manoranjan, Mishra [22]) In short, 10mM pyrogallol solution was prepared in 25mM phosphate buffer (pH 6.8). To 5ml of the above solution, 50 µl of ten times diluted 30% H2O2 and 10 µl of enzyme extract were added. Absorbance was recorded at 420 nm for 4 mins. The POD activity of the leaf extract is measured by the conversion of pyrogallol to purpurogallin in the presence of hydrogen peroxide. One unit of enzyme activity is the formation of 1 µmol purpurogalin per minute.
Beyer and Fridowich's (Beyer Jr, Fridovich [23]) method was used to measure Superoxide dismutase (SOD; EC 1.15.1.1) spectrophotometrically. The enzyme extracts were incubated at 25°C under light for 10 min in 50 mM sodium phosphate buffer (pH 7.8) containing 10mM L-methionine, 33 µM NBT, .66 mM EDTA, and 0.0033 mM riboflavin. The activity was measured at 560 nm. One unit of SOD activity was expressed as the quantity that reduces the photochemical activity of NBT to 50%. Catalase (CAT; EC 1.11.1.6) activity was measured according to Bergmeyer (Bergmeyer [24]). The activity was assayed in the reaction mixture containing 0.05 M Na phosphate buffer (pH 7, 0.1 mM EDTA) and 3% H2O2. The disappearance of H2O2 was measured at 240 nm. One unit of CAT activity was defined as 1 µmol H2O2 destroyed per minute.
Effect on Chlorophyll Content: The content of chlorophyll a (Chl a), chlorophyll b (Chl b), and total chlorophyll content were determined by the formula given by Harborne (Harborne [25]). Fresh leaves were cut into small pieces and 0.2g was macerated in 80% acetone. The samples were preserved at 4°C for 24hrs in dark. The mixture was adjusted to 6.25 ml with 80% acetone, centrifuged and then filtered with Whatman No.41 filter paper. Chlorophyll content was measured in a UV-vis spectrophotometer (T70/T80 series UV/Vis spectrophotometer PG Instrument Ltd., England) at 663 nm for (Chl a) and 645nm (Chl b). Chlorophyll a, b, and total chlorophyll content were calculated using the given formula.

Where A-absorbance, P sample weight in g, V volume, C concentration in mg, 1000 conversion factor.
Statistical Method
Statistical analysis like arthematic mean, and standard deviation for comparison between more than two groups with different parameters ANOVA-test was done.
Synthesis and Characterization of Silver Nanoparticles
Uv-vis Spectrophotometer: As the teak leaf extract was added to 1 mM silver nitrate solution the colour changed from faint red to deep red to reddish brown indicating the formation of silver nanoparticles (Figure 2). The UV-Vis spectra were recorded at time intervals of 30, 45, 60 min, 24 hr, and 48 hr are shown in Figure 3. Absorption spectra of silver nanoparticles showed absorption maxima at 444 nm due to Surface Plasmon resonance of silver nanoparticles.
Figure 2 Change in colour of the plant extract containing AgNP after 30min, 45min, 60min, 24hr, 48hr.

DLS Analysis: The particle size of the biogenic silver nanoparticle was determined by the particle analyzer. Z-Average (d.nm) was 85.62nm with 80% peak intensity (Figure 4).
Effect of Silver Nanoparticles on Seed Germination
Toxicity study of silver nanoparticles was done by observing the germination percentage (%G) and relative germination (%RG) of Vigna radiata seeds exposed to different concentrations of nanoparticles. Emergence of radicles confirms the germination of seeds and maximum germination was seen after 24hrs. The percentage of germination in case of control seeds was 90% and vigor index was 621. Germination percentage for 5 ppm, and 10 ppm treated AgNPs was 91% and 92% respectively. Highest germination percentage for 20 ppm treated AgNP was 95% and vigor index 760 (Figure 5).
Effect of Silver Nanoparticles on Root and Shoot Length
Root and shoot length and biomass were used to evaluate the dose dependent variation of AgNPs on the growth parameters of mung seedlings. The effect of AgNPs on the root and shoot of mung seedlings are shown in Figures 6a & 6b. It was observed that with increase in AgNP concentration, the root and shoot growth also increased. The results of root and shoot length validated that AgNPs treatments (5 ppm, 10 ppm, and 20 ppm) significantly increased the length with increasing concentrations of AgNPs. On the third day of treatment, there was not much difference in root elongation between control and treated seedlings. Fifth day onwards a significant increase in the root length was 2.2 cm ± 0.02 at 5 ppm, 2.75 cm±0.06 at 10 ppm, and 2.84 cm ±0.03 at 20ppm while for control it was 1.8cm±0.02. The root length of plants after treatment was 4.02cm±0.08 at 5ppm, 4.76 ± 0.04 at 10ppm, 4.86±0.02 at 20ppm and 4.01±0.18cm for control seedlings. On the 11th day of treatment 24.3%, 26%, and 15.7% increase in root length for the treated plants for 5ppm, 10ppm, and 20ppm respectively. On the 15th day of treatment highest increase in root length 21.25% was noted for 20 ppm treated AgNPs, and 19% for 10 ppm treated AgNPs. The best growth value for mung seedlings was at 20 ppm, with an increase of 21.25% (P value 5.6x10-6).
Figure 6 Dose dependant variation in V.radiata seedlings in the (a)root length (b) root biomass during the study period of 15 days (p<0.05),. The values were given as mean ± SD (standard deviation) of triplicate samples.
The shoot length in control and treated Vigna radiata plantlets did not show any significant change up to the third day of treatment. On the third day, the control shoot length on average was 5.7±0.12 cm while the 5 ppm treated shoot plant and 10 ppm AgNP treated shoot plant was 5.9 cm. The 20ppm treated AgNP showed a maximum length of 6.2±0.23 cm. On the fifth-day average control shoot length was 7.3±0.23 cm, and 5 ppm treated AgNP was 8.5±.17 cm, 10 ppm treated AgNPs was 8.8±0.22 cm and highest observed shoot length was for 20 ppm treated AgNP 9.2±0.56 cm. A similar growth pattern of the shoot was observed on seventh and eleventh days. The control plant shoot length was lowest throughout the study period of 15 days. The average shoot length of the control plant on the 15th day was 12.45±0.23 cm, 5 ppm treated AgNP it was 14±0.35 cm, 10 ppm treated AgNP shoot length was 15.23±0.45 cm, and 20 ppm treated AgNP it was 15.75±0.13 cm. The 22.3% increase in shoot length (P value 1.3 x 10-4) of 20 ppm treated seedlings was the highest compared to 14.23% and 12.45% for 5 ppm and 10 ppm respectively (Figure 7).
Figure 7 Percentage increase in shoot of mung seedlings as compared to control during the experimental period of 15days (p<0.05).

Effect of Nano-silver Particles Suspension on Biomass
The fresh mass of shoots and roots of seedlings treated with AgNPs was higher than those grown in normal conditions (control). The fresh weight of the shoot and root did not show any significant change till the third day of treatment. On the fifth day of treatment, there was no significant increase in the root biomass for 5 ppm and 10 ppm treatment but a 20% increase in root biomass (P value 0.005) was recorded for 20 ppm with respect to control. On the 5th day of shoot biomass for 5 ppm and 10 ppm treated seedlings increased by 45% and 60% respectively while the maximum increase in shoot biomass was seen at 20ppm 68% (P value 1.74 x 10-11). On day 7th and day 11th, a steady increase in shoot biomass was recorded for all the treatment seedlings. Similarly, a steady increase in root biomass with respect to control was observed for all the AgNPs treated seedlings. On the 15th day, a significant increase in shoot biomass 13.8% (P value 0.025) and 20.6% (P value 0.0041) was observed at 5 ppm and 10 ppm treatment respectively. Similarly, a significant increase in root biomass 15% (P value 0.004) and 20% (P value 0.0003) was observed for 5 ppm and 10 ppm treatment respectively. The maximum increase in shoot biomass was 31% (P value .001) and root biomass was 30% (P value 3.04x 10-5) (Figure 8). This showed that AgNPs present at lower concentrations could boost root and shoot growth.
Figure 8 Percentage increase in root and shoot biomass at the end of experimental period of 15 days.

Change in Chlorophyll Content on Exposure to AgNPs
Effect on total chlorophyll content of Vigna radiata seedlings exposed to a varied concentration of AgNPs was recorded. The chlorophyll content of 5 ppm, 10 ppm, and 20 ppm plants was the same as the control till the 3rd day. On the 5th day total chlorophyll content was 1.49±0.23 mg/g of FW for control, 1.58 ± 0.17 mg/g of FW for 5 ppm, 1.5± 0.38 mg/g of FW for 10 ppm and 1.54± 0.08 mg/g of FW for 20 ppm showing significant upregulation (p=0.047). After the 7th day of treatment, a significant increase in the chlorophyll content of AgNP treated 20 ppm (p=0.02) plants was observed. On the 11th and 13th day total chlorophyll content was maximum in 20ppm treated seedlings as compared to control and 5 ppm and 10 ppm treated plants (Figures 9a & 9b). On the 15th day of treatment, Vigna radiata treated seedlings showed a significant increase in total chlorophyll content 2.09 mg/g of fresh weight as compared to 1.82 mg/g of fresh weight of control.
Figure 9 A. Effect of Ag nanoparticles on total chlorophyll content of V.radiata 15 days of treatment. B. Effect of Ag nanoparticles on chlorophyll ratio of V.radiata seedlings after 15 days of treatment

Effect on Protein Concentration
Total protein level was found to be higher in AgNP treated Vigna radiata compared to the control. The result showed a dose dependent increase of protein concentration in the seedlings of Vigna radiata. At 5 ppm 13% increase in protein concentration over control (P value 0.0002) and at 10 ppm 21.7% increase in protein concentration over control was recorded (P value 2.78x10-5). Maximum increase in protein concentration was recorded at 20 ppm 30.4% over control and P value 8.8x 10-6. These findings suggest that different concentrations of AgNPs have a significant impact on protein concentration (Figure 10).
Figure 10 Impact of various concentrations of AgNPs total protein content. Data are average ± standard errors of experiments performed in triplicate.

Effect on Antioxidant Enzymes
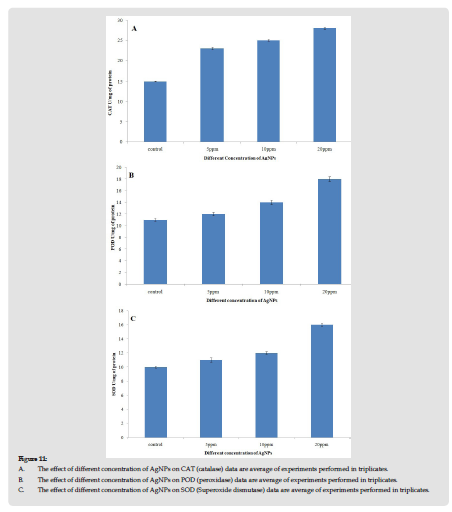
The anti-oxidant enzymes (SOD, POD, CAT) of V. radiate leaves were over-expressed after the treatment of AgNPs. POD activity was increased significantly with respect to control. At 5 ppm exposure, it was 15 U/mg of protein and 28U/mg (p<0.05) of protein. A significant increase in the Catalase enzyme activities was seen for all the treatments (Figure 11a). A gradual increase in peroxidase activities was seen for the three treatments 1.63 folds, 1.27 folds, and 1.09 folds for 5 ppm, 10 ppm, and 20 ppm respectively (Figure 11b). A significant increase in SOD activity was seen in the seedlings of all the three treatments as compared to control (Figure 11c). Increase in antioxidant enzymes activities was higher for all the studied enzymes in 20 ppm treated AgNPs. CAT activity was highest with a 1.87 folds increase, SOD 1.6 folds and Peroxidase 1.64-fold increase when compared to control.
Figure 11 A. The effect of different concentration of AgNPs on CAT (catalase) data are average of experiments performed in triplicates. B. The effect of different concentration of AgNPs on POD (peroxidase) data are average of experiments performed in triplicates. C. The effect of different concentration of AgNPs on SOD (Superoxide dismutase) data are average of experiments performed in triplicates.
Biomass Assay by ICP-AES
AgNPs concentration in root and shoot of mung bean seedlings is shown in table 1. It was seen that with an increase in the concentration of AgNPs, the concentration in root and shoot increased. It indicates that AgNPs are adsorbed and translocated to the upper parts of the seedlings.
The impact of nanoparticles on plants can be determined by their effects on different physiological parameters like seed germination, vigor index, root length, shoot length, biomass, chlorophyll content, protein content, and stress response. Metal nanoparticles can enter the soil either through treated sewage in the land application or through the use of nano pesticides and nano fertilisers. The influence of nanoparticles on the physiological indices of plants can be negative and positive. Several factors like size of the nanoparticle, coating material, hydrophobicity, concentration, mode of application, suspension medium, and sunlight control the toxicity of nanoparticles. Similarly, nanoparticles uptake and translocation can vary amongst plants because of the variety of physiological and morphological features (Monica, Cremonini, et al. [26,27]). The various mechanisms by which nanoparticles enter the inside of plant tissues is complex multidimensional interaction between soil particles, part of plant that is exposed to nanoparticles like leaf, seeds, roots, etc and the vascular morphological diversities in the plant types. The growing use of AgNPs in various agricultural products such as plant growth stimulators (Monica, Cremonini [26]), fungicides (Alavi, Dehpour [28]), and fruit ripeners (Vinković, et al. [29]) increases its chance to enter the food chain thus posing a potential threat to humans (Cvjetko, et al. [30]).
Green Synthesis of Silver Nanoparticles and Its Characterization
Silver nanoparticles can be synthesized through physical, chemical, and biological methods. Green synthesis of silver nanoparticles can be done by using microbes like bacteria, yeast, actinomycetes or plant extracts. Biogenic synthesis can be done by utilizing any plant parts like; the use of leaves of Azadiracta indica (Roy, et al. [14]), bark extract of Saraca asoca (Banerjee, Nath [31]), extracts of fruits of the fruit of Malus domestica (Roy, et al. [14]), hibiscus flowers (Philip [32]), plant extracts of Aloe vera (Chandran, et al. [33]), seed extract of Jatropha (Bar, et al. [16]) to name a few. Teak leaves are a storehouse of bioactive compounds such as Quinones, Steroidal compounds, Apocarotenoids, Flavonoids, Glycosides, Phenolic acids, Saponins, Proteins, and dyes (Kore, et al. [34]). In the current study, aqueous teak leaf extract was used for the synthesis of silver nanoparticles. A change in colour from pale red to deep brown confirms the synthesis of silver nanoparticles which is attributed to the reduction of silver precursor salt (AgNO3) to AgNPs by the bioactive compounds present in the teak extract that act as a capping and stabilizing agent. Further to confirm the formation of AgNPs, biosynthesized nanoparticles were analyzed using UV-Visible spectroscopy. The optical properties of plasmonic nanoparticles like silver depend upon its concentration, shape, size, agglomeration rate, and refractive index. This makes UV-Vis Spectroscopy an important method for characterizing, analyzing, and identifying nanoparticles. Absorbance of AgNPs in the aqueous solution is studied in the range between 400 to 445 nm and is dependent on the reducing agent (plant extract) that is used for biogenesis. Surface Plasmon Resonance (SPR) is due to the oscillation of electrons around the surface of nanoparticles. In the current study surface Plasmon peak was at around 444nm and the peak intensities increased with time confirming the synthesis of AgNPs. An increase in concentration of AgNPs and as the intensity of SPR peaks narrows it reveals particles in suspensions are monodispersed (Khalil, et al. [35]) Similarly during the biogenesis of AgNPs using T.grandis seed extract a maximum absorption was obtained at 440nm and the intensity of peak increased with time (Rautela, Rani [36]). The formation of AgNPs was further confirmed by DLS.
Effect on Seed Germination, Plant Biomass, Root, and Shoot Length
After exposure to AgNPs, momentous alterations were seen in the growth potential, seed germination, plant biomass, and root and shoot length as compared to the control. In the current study green synthesized AgNPs had a marginal effect on seed germination. Similarly in a study on the impact of AgNPs on the growth parameters of three plant species, watermelon, corn, and zucchini; germination rates were enhanced in response to AgNPs (Almutairi, Alharbi [37]). In tomato (Solanum lycopersicum), 5000 mg/L of AgNP did not affect germination (Song, et al. [38]). The phytostimulatory effect of biosynthesized AgNPs during seed germination and plant growth of Oryza sativa has also been reported (Gupta, et al. [39]). Karimi, et al. (Karimi, et al. [40]) observed that AgNP application on wheat seed did not affect its germination. Furthermore, germination in seeds treated with AgNPs might be enhanced due to nanoholes in the seed coat so that they can enter the seeds easily (Khan, et al. [41]). The second reason might be the slow and reduced release of silver ions (Ag+) that lowers the deleterious effects of AgNPs to a great extent. Again permeation, collection, and dispersion of AgNPs are primarily dependent on the shape, size, concentration, and plant types (Parveen, Rao [42]). In the current study size of AgNP was big 85nm and much lower concentration AgNPs were used 5 ppm, 10 ppm, and 20 ppm. This probably might be less harmful in comparison to an AgNP that is much smaller in size and higher in concentration. It has been reported in previous studies that nanoparticles can enhance seed germination by increasing water absorption by the seeds (Zheng, et al. [43]), utilizing water and fertilizer, and activating the seed antioxidant system. Further nanoparticles reduce stress by lowering H2O2 (Changmei, et al. [44]), superoxide radicals, and malondialdehyde content and stimulating the release of many enzymes such as superoxide dismutase, ascorbate peroxidase, and catalase (Lei, et al. [45]). Both mechanisms are known to improve seed germination in many plant species.
Silver nanoparticles acting as nanocarriers are known to enhance plant growth and stress tolerance. The impact of AgNPs on plant growth, root, and shoot elongation, and biomass accumulation depends on their physicochemical properties (size, coating agent, shape), the application method (soil, hydroponics, seeds, foliar spray), and concentration. In the current study, an overall increase in root and shoot length and plant biomass of Vigna radiata was observed as compared to the control. All the tested concentrations of AgNPs (5 ppm, 10 ppm, 20 ppm) promote both root and shoot growth that was validated by the increased length and biomass of the seedlings as compared to the untreated plant. It has been reported that when corn and common bean were treated with 60 ppm or higher concentrations of AgNPs the root length considerably decreased however when treated with a lower concentration then root length elongation was observed (Almutairi, Alharbi [37]) which is very much similar to the current study. In the present report also 20ppm treated AgNP showed maximum elongation in the root length as compared to the control. Maximum growth of the seedling and biomass accumulation was seen in the 20ppm concentration of AgNP. The phytostimulatory effect of biosynthesized silver nanoparticles has been reported in the seedling growth of rice (Oryza sativa L., cv. Swarna) in all tested concentrations of AgNPs (10, 20, 40 ppm) (Gupta et al. [39]). In B. juncea seedlings dose dependant (50ppm) stimulatory effect of AgNP resulted in a 326% increase in root length and a 133% increase in vigor index (Sharma, et al. [46]). AgNPs increased plant growth and other biochemical features in many other plant types like onion (Acharya, et al. [47]), Brassica juncea (Sharma, et al. [46]), common beans, corn (Salama [48]), and rice (Mahakham et al. [49]).
In the present study nanoparticle-treated Vigna radiata seeds grew well compared to the untreated control. It can be speculated that AgNPs accumulated in the seeds might trigger many metabolic pathways that are essential for seed germination, root and shoot growth. The transport of nanoparticles through phloem can modify gene expression (Khodakovskaya, et al. [50]) resulting in an overall increase in the physiological indices of the plant. Another important property that controls the phytotoxicity of silver nanoparticles is the size of AgNPs. Smaller size silver nanoparticles have a large surface-to-volume ratio, which can react better with the cell membrane. Plant cell walls act like natural sieves and AgNPs have to diffuse through the cell wall and plasma membrane to enter the vascular system to reach the leaves. Thus it has been proved by various experiments that smaller AgNPs can accrue in any plant tissue and be more toxic than their bulky counterparts. In Arabidopsis AgNPs of smaller dimension was found to accumulate more in the seedlings as compared to larger AgNPs (Geisler-Lee, et al. [51]). Various phytotoxicity studies using different sizes of AgNPs have shown that size of AgNPs is negatively correlated to toxicity (Cvjetko et al. [30,52]).
Effect of Silver Nanoparticles on Chlorophyll
Chlorophyll content and other macronutrients can act as important biomarkers of plant health (Dutta Gupta, Pattanayak [53]). Increased synthesis of chlorophyll and more pronounced uptake of minerals will increase plant growth and overall biomass production. In the current study mung seeds grown in 20 ppm, AgNPs showed a maximum increase in chlorophyll content as compared to 5 ppm, 10 ppm, and control mung bean seedlings. A consistent increase in chlorophyll a was seen throughout the 15 days of the study. Similarly, total chlorophyll along with carotenoids increased under the influence of AgNP in rice seedlings (Dutta Gupta, Pattanayak [53,54]) Upregulation in the chlorophyll synthesis in leaves of Triticum aestivum was seen after the foliar application of AgNPs (Latifm et al. [55]). Applying colloidal suspension of AgNPs on pelargonium plants increased chlorophyll and carotenoid content (Hatami, Ghorbanpour [56]). AgNPs are assumed to affect the gene expression profiles by ultimately modifying physiological, and biochemical pathways in plants. In Phaseolus vulgaris after treatment with AgNPs an increase in chlorophyll content, escalation in iron and nitrogen uptake, protein accumulation, and upregulation in mRNA for nitrate reductase and eferredoxin is seen (Das, et al. [57]). Brassica juncea seedlings treated with AgNPs showed a higher concentration of total chlorophyll as compared to the control (Sharma, et al. [46]).
AgNPs enter the plants through nutrient and water uptake. The cell wall has many pores through which small-sized nanoparticles enter the plasma membrane and are carried by the vascular system to the leaves but larger nanoparticles are sieved out (Ma, et al. [58]). However, sometimes, AgNPs can create new pores which allow the diffusion of larger AgNPs through the cell wall (Navarro, et al. [59]). AgNPs can be translocated through endocytosis, and vesicles carrying the AgNPs are then transferred from the plasma membrane to the cells (Fabrega, et al. [60]). AgNPs translocate in the plants via the intercellular spaces through the plasmodesmata and are gradually distributed in the shoot reaching the leaves. AgNPs used in low doses have a phytostimulatory effect and it varies depending on the type of plant species. It can be speculated that AgNPs inside the leaves can induce chlorophyll synthesis by either accruing proteins associated with the cell cycle and carbohydrate metabolism or by changes in the expression of genes controlling photosynthesis, cellular proliferation, or the signalling pathway of hormones. Increase in accumulation of chlorophyll upregulates the photosynthesis process in leaves as suggested by previous researchers (Gupta, et al. [39,61]). So in this study, we did not observe any phytotoxic effect of AgNPs.
Analysis of Total Protein Content and Oxidative Stress
Several studies have shown that AgNPs induce oxidative stress in plants by generating ROS (Reactive Oxygen Species) like superoxide radical, hydroxyl radical, hydrogen peroxide, and singlet oxygen (Kumar, et al. [62]). They cause lipid peroxidation, and membrane damage affecting macromolecules like protein-nucleic acids ultimately leading to cell death. Plants have many tolerance mechanisms by which they can counter the ROS like the upregulation of genes expressing antioxidant enzymes (Apel, Hirt [63]). There are many enzymatic scavengers in plant cells like APX, SOD, and CAT that can protect the cells from stress (Nair, Chung, 2014). Due to increase in activities of oxidative enzymes plants can fight oxidative stress. In the current study elevated levels of SOD, CAT, and POD were observed for AgNPs treated seedlings. It has been reported in various studies that these enzymes protect against cell membrane damage in AgNPs treated seedlings (Sharma, et al. [46,64]). Mehta, et al. [65] revealed that AgNP did not have harmful effect on wheat seedlings while the application of AgNPs on brassica boosted its early growth by controlling oxidative stress (Sharma, et al. [46]). AgNPs treated O.sativa roots showed an increase in SOD, APX, and glutathione S-transferase proteins (Mirzajani, et al. [54]). Similarly in Pisum sativum seedlings AgNPs SOD and APX activities were elevated and GR (glutathione reductase) and DHAR activity were reduced (Durgesh Kumar Tripathi, et al. [66]). Apart from CAT, an increase in POD level may check lipid peroxidation, thus protecting the seedlings from damage due to AgNPs. A gradual increase in SOD activity was seen at treatment with the increase in the concentration of AgNPs. The antioxidative mechanism in the V. radiata seedlings indicated a balance between scavenging of ROS and generation of antioxidant enzymes by stimulation of AgNPs. Plant growth and development are impaired when ROS homeostasis is disrupted, while maintenance of ROS levels within acceptable parameters can enhance plant growth (Mittler [67]). In contrast to phytotoxicity studies on AgNPs, the current study reports the phytostimulatory effects of AgNPs. The low level of ROS could be attributed to the efficient ROS scavenging mechanism induced by AgNPs increasing photosynthesis and plant growth.
At the molecular level, several genes that are part of antioxidant defense mechanism are upregulated as a stress response to AgNPs. An increase in the concentration of antioxidant enzymes such as SOD, catalase, and peroxidase will contribute to the increase in protein concentration. AgNPs when applied in soil substrate for the growth of Phaseolus vulgaris have increased chlorophyll content, nitrogen and phosphorus uptake, upregulation of mRNA for nitrate reductase, eferredoxin and accumulation of crude protein (Das, et al. [57]). Similarly, the phytostimulatory activity of AgNPs in Arabidopsis has been attributed to the accumulation of proteins involved in carbohydrate metabolism, cell cycle, and in the expression of various proteins involved in multicellular processes like cell proliferation, photosynthesis, signaling pathways of hormones and antioxidant enzymes (Syu, et al. [68]). In the current study an increase in protein concentration was observed for 20 ppm AgNP treated mung seedlings. It can be assumed that the increase in protein concentration is directly dependent on the increase in plant biomass which is perhaps due to the over-expression of many stress-regulating genes and enzymes involved in several metabolic pathways.
Bioaccumulation of AgNPs in the Root and Shoot
Uptake of AgNPs depends upon the plant species, concentration, and size of AgNPs. In this study as the concentration of AgNPs increased in the medium it absorption rate also increased and maximum concentration in root and shoot was observed for 20ppm treated AgNPs. This observation is contradictory to some of the previous reports, that dose concentration determines the bioaccumulation of AgNPs (Rahmatpour, et al. [69]). Proximity of AgNPs to the roots, it suggests their uptake and higher accumulation in roots. The release of metal ions is an inevitable outcome when nanoparticles are used as crop growth stimulants. However many reports have shown that the metal ions that are released do not have much impact on plants, compared to their nanoparticles counterparts in suspensions.
During the past decade, several researches have focused on the interaction and impact of AgNPs on crops. Most of these studies have highlighted the detrimental effect of AgNPs at physiological, biochemical, cellular, and genetic levels. However, a few studies have also reported the positive effect of AgNPs on plant growth and development. These contradictory reports suggest that the interactions are not only dependant on various properties of AgNPs (size, shape, concentration, capping, and stabilizing agent, engineered on green synthesized nanoparticles), but are also determined by plant system used (species, organ, developmental stage) and application method of AgNPs (medium, soil, foliar spray, time of exposure, etc) (Yan, Chen [70]). In response to AgNPs, the plants can activate enzymatic and nonenzymatic pathways for detoxification. Some plants may upregulate antioxidant enzymes like catalase and peroxidase to reduce stress-induced due to metal nanoparticles. In this study stress induced due to ROS was modulated by elevated levels of SOD, CAT, and POD. This lowering of ROS might have stimulated plant growth and development. An increase in total chlorophyll content and total protein content also validates that 20 ppm concentration of AgNPs stimulates plant growth [71,72].
The phytotoxicity of AgNPs at a cellular level and genetic level has to be understood to predict the exact tolerance mechanism in plants. Further studies have to be done to predict the lethal/sub-lethal or optimum concentration of AgNPs that can be applied to various plant species so that a regulatory framework could be made. The translocation of AgNPs in the food chain can have harmful effects on man. So the proper understanding of tolerance mechanisms in plants and toxicological studies in humans have to be analyzed to harness the beneficial effects of AgNPs on the ecosystem.
The authors declare that they have no competing interests.
The authors are thankful to the Department of Biotechnology, College of Basic Science and Humanities, OUAT Bhubaneswar.
Conceptualization, K. Das and P. Roy; Investigation, S. Champatiray and M.Jena.; Supervision, K Das, CSK Mishra, and P.Roy.; Validation, K. Das, C.S.K. Mishra, and P. Roy; Writing original draft, P. Roy, S. P. Panda, Writing-review and editing, P. Roy. All authors have read and agreed to the published version of the manuscript.


