Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Ibrahim Sadik1*, Megersa Bedassa2 and Ephrem Shimelis3
Received: June 14, 2023; Published: June 20, 2023
*Corresponding author: EV Husakouskaya, Grodno State Medical University, Department of Pathophysiology named after D. A. Maslakov, Belarus, Grodno, Gorkogo Street, Belarus
DOI: 10.26717/BJSTR.2023.51.006053
Background: Incomplete understanding of peritonitis pathogenesis may be the cause of slow tendency to decrease in mortality in this pathology.
Objective: To study the activity of «L-arginine-NO-system», severity of oxidative stress and state of endothelium and peritoneum in rodent peritonitis.
Material and Methods: The experiments were carried out on male rats (n=36), divided into 2 equal series, which were injected intraperitoneally with: 1st series (control) – 0,9% sodium chloride, 2nd series (experimental peritonitis, EP) – 15 % fecal suspension, 0,6 ml/100 g. Studies were made after half a day (n=6), one day (n=6) and three days (n=6) of EP while an assessment of structural changes in the peritoneum were carried out after half a day and 3 days of EP. The level of nitrates/nitrites (NOx), the activity of oxidative stress by measurement of the lipid peroxidation product, malondialdehyde, and an indicator of antioxidant protection, reduced glutathione, in blood plasma (BP) and peritoneal fluid were determined, along with the estimation of damage to endothelium of blood vessels basing on number of circulating endothelial cells in BP, and alteration of the peritoneum in preparations of the anterior abdominal wall and ileum stained with hematoxylin and eosin.
Results and Discussion: During all the studied periods of EP, an increase in content of malondialdehyde and decrease in concentration of reduced glutathione were observed in BP and peritoneal fluid of rats, indicating development of oxidative stress, and elevation of number of circulating endothelial cells in BP, which corresponded to an unidirectional change in the level of NOx and could indicate a relationship between activation of inducible NO-synthase isoform, changes in prooxidant-antioxidant state and damage to endothelium of blood vessels. Revealed inflammation of the visceral and parietal layers of the peritoneum served as morphological substrate of acute EP, which was manifested by disorder of its structure, leukocyte infiltration and changes in microcirculation.
Conclusion: The study of the fecal peritonitis course in rats revealed: an increase in the content of NOx indicating enhance in NO production, as well as an increase in concentration of the lipid peroxidation product and a decrease in the level of antioxidant protection in blood plasma and peritoneal fluid, characterizing the development of oxidative stress; pronounced desquamation of endotheliocytes from the blood vessels wall, indicating the endothelial dysfunction; significant disorder of the peritoneum structure; development of changes in the studied parameters in all periods of experimental peritonitis to a greater extent in the peritoneum and peritoneal fluid than in blood, reflecting development of inflammation at the local level.
Keywords: Peritonitis; Nitrates/Nitrites; Oxidative Stress; Endotheliu; Peritoneum; Rodents
Abbreviations: BP: Blood Plasma; PF: Peritoneal Fluid; MDA: Malondialdehyd; CEC: Circulating Endothelial Cells; NOS: NO-synthase; NO: Nitric Oxide
Diffuse peritonitis remains one of the urgent problems of surgery due to the persistent high mortality rate, which reaches 85-90% with the development of septic shock and multiple organ failure [1-3]. At the same time, the most numerous group of patients with peritonitis is a working age group [4], and its treatment requires significant economic expenses, which are much more higher than the expenses for treatment of diseases not accompanied by infectious complications [5]. These data indicate pronounced socio-economic significance of peritonitis. Surgery is an obligatory standard in the treatment of diffuse peritonitis and is always combined with antibiotic therapy, which is quite well developed [6]. However, a high mortality may be due to inferiority of the pathogenetic therapy of peritonitis because of insufficient understanding of the mechanisms of its development. Until now, the mechanisms of the oxidative stress and microcirculatory disorders development and damage to peritoneum in peritonitis remain incompletely studied, while the influence on them can improve the prediction in this pathology. Objective of the research was to study the activity of «L-arginine-NO-system», severity of oxidative stress and state of endothelium and peritoneum in rodent fecal peritonitis.
Experiments were carried out on outbred male rats, 230-250 g (n=36), in accordance with the Helsinki Declaration on the Humane Treatment of Animals (1964). Rats were divided into 2 equal series, which were injected intraperitoneally with: 1st series (control) – 0,9% sodium chloride, 2nd series (experimental peritonitis, EP) – 15 % fecal suspension, 0,6 ml/100 g of body weight, according to the modified method described by V.A. Lazarenko, et al. [7,8]. The suspension was standardized according to the extinction coefficient and the number of bacterial cells [8]. In each series, studies were carried out after half a day (n=6), one day (n=6) and three days (n=6) after peritonitis modeling, and an assessment of structural changes in the peritoneum were carried out after half a day and 3 days of EP. The level of NOx was determined in blood plasma (BP) and peritoneal fluid (PF) using the Griess reagent and cadmium on a «SOLAR PV 1251C» spectrophotometer [9]. The activity of oxidative stress was assessed by the concentration of lipid peroxidation product, malondialdehyde (MDA), and an indicator of antioxidant protection, reduced glutathione (GSH), in BP and PF. The content of MDA was determined by measuring the extinction of a solution containing the trimethyl complex of MDA with thiobarbituric acid, and determination of [GSH] was performed using trichloroacetic acid and Ellman's reagent during spectrophotometry [10].
The state of the endothelium of blood vessels was assessed by counting the number of circulating endothelial cells (CEC) in BP with use of hemocytometer [11]. Morphological changes of peritoneum were determined in preparations of the anterior abdominal wall and ileum stained with hematoxylin and eosin under light microscopy using a Micromed 3 var 3-20M microscope (China) equipped with a RisingCam E3CMOS 20000KPB digital video camera (China), with using the software «RisingView» for microphotography. Changes in the structure of the peritoneum were characterized using a scale for semi-quantitative assessment of disorders (from + to ++++). Statistical data processing was performed using the Statistica 10.0 program for Windows (StatSoft Inc., USA) after checking for normal distribution (Shapiro-Wilk test) using the nonparametric Kruskal-Wallis test and post hoc comparisons; data are presented as Me (LQ; UQ), where Me is median, LQ and UQ are values of the lower and upper quartiles, respectively. Differences were considered statistically significant at p<0.05 [12].
The EP development in rats was characterized by an increase in the content of NOx in the BP and PF after half a day – 5.6 times (p<0.01) and 12.2 times (p<0.01), after 1 day – 6.6 times (p<0.01) and 15.0 times (p<0.01), after 3 days – 4.0 times (p<0.01) and 9.9 times (p<0.01), respectively (Table 1), indicating a significant increase in NO production by inducible NO-synthase (NOS) isoform after its stimulation with bacteria and inflammatory cytokines [13]. At the same time, [NOx] in BP and PF after 1 day of peritonitis was more than after half a day, 1.2 times (p˂0.05) in both studied media, and after 3 days – less than after half a day, 1.4 times (p<0.05) and 1.2 times (p<0.05), and [NOx] after 3 days of EP was less than after 1 day – 1.6 times (p<0 .05) and 1.5 times (p<0.05) respectively, reflecting the maximal increase in inducible NOS activity by day 1 and its decrease by day 3 of EP. This may be due to both depletion of the NOS substrate, amino acid L-arginine [14], and decrease in activity of inflammation. It is important to note that the content of NOx in PF was significantly higher than in BP, that shows high intensity of inflammatory process locally, in the abdominal cavity. Along with an increase in the NOx level in rats with peritonitis, rise in concentration of the lipid peroxidation product, malondialdehyde (MDA), in BP and PF after half a day of EP was found 4.7 times (p<0.01) and 9.8 times (p<0.01), after 1 day – 6.1 times (p<0.01) and 11.8 times (p<0.01), after 3 days – 4.4 times (p< 0.01) and 9.0 times (p<0.01) respectively, which indicates the development of oxidative stress in all studied periods.
Table 1: Indicators of nitric oxide production and prooxidant-antioxidant state in rats with experimental peritonitis (EP), Me (LQ; UQ).
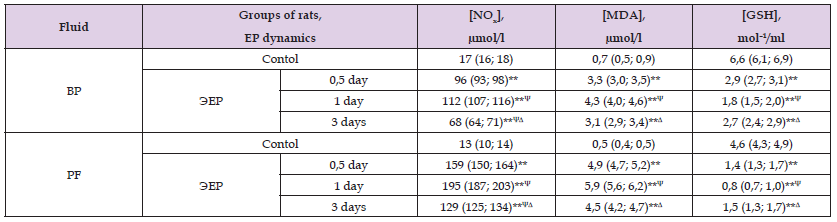
Notes: NOx – nitrites/nitrates, MDA – malonic dialdehyde, GSH – reduced glutathione, BP – blood plasma, PF – peritoneal fluid, significant differences relative to: *– p<0,05, **– p<0,01, ***– p<0,001 – control group; Ψ- p<0,05 – half a day and Δ– p<0,05 – 1 day within the group.
At the same time [MDA] in BP after 1 day was more than after half a day, 1.3 times (p˂0.05) in the absence of changes in PF (p˃0.05), and after 3 days in BP and PF less than after 1 day, 1.4 times (p˂0.05) and 1.3 times (p˂0.05) respectively, when after 3 days the values of the indicator didn’t differ from the values after half a day, that shows the greatest intensity of oxidative processes after 1 day of peritonitis. At the same time, there was a decrease in the content of the antioxidant, reduced glutathione (GSH) in BP and PF after half a day of peritonitis – 2.3 times (p<0.01) and 3.3 times (p<0.01), after 1 day – 3.7 times (p<0.01) and 5.8 times (p<0.01), after 3 days – 2.4 times (p<0.01) and 3.1 times (p<0.01 ) respectively. The level of GSH in BP and PF of rats after 1 day was less than after half a day, by 1.6 times (p˂0.05) and 1.8 times (p<0.05) respectively, and after 3 days was more than after 1 day, 1.5 times (p<0.05) and 1.9 times (p<0.05) respectively, while after 3 days [GSH] didn’t differ from the values in half a day of peritonitis in both studied media (p˃0.05), which indicates the maximum inhibition of antioxidant protection after 1 day of inflammation. Thus, the development of EP was accompanied by an increase in severity of lipid peroxidation processes and inhibition of antioxidant protection, which can be due to participation of leukocytes in formation of reactive oxygen species in neutrophils with participation of NADPH2-oxidase, myeloperoxidase and nitric oxide (NO) in macrophages with the participation of inducible NOS isoform, subsequently converted to peroxynitrite (ONOO-).
Figure 1 1: The content of circulating endothelial cells (CECs) in blood of rats with experimental peritonitis (EP).
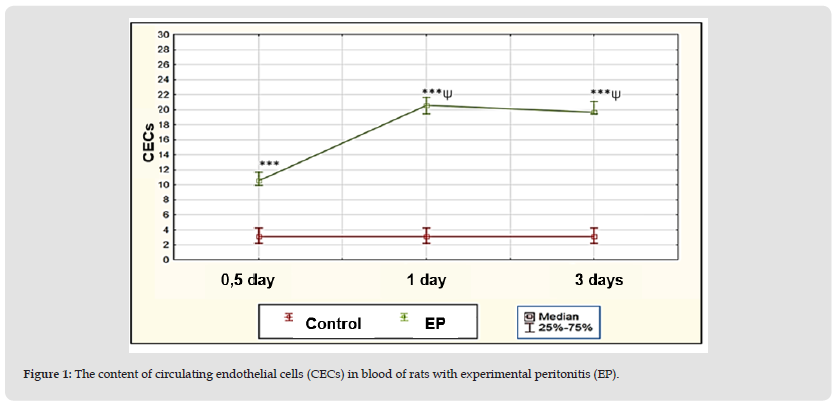
Note: Significant differences relative to: *– p<0,05, ** – p<0,01, ***– p<0,001 – control group; Ψ– p<0,05 – half a day and Δ– p<0,05 – 1 day within the group.
In addition, in rats with EP, an increase in number of CECs in blood as an indicator of damage to vascular endothelium (Figure 1) was found after half a day – up to 10.6 (10; 11.7)/μl, or 3.4 times (p<0.001 ), after 1 day – up to 20.6 (19.4; 21.7)/µl, or 6.6 times (p<0.001), after 3 days – up to 19.7 (19.4; 21.1 )/µl, or 6.4 times (p<0.001), compared with the value in the control group – 3.1 (2.2; 4.2)/µl, which may be caused by «bacterial aggression» and oxidative damage [15]. At the same time, the number of CECs after 1 day and 3 days of peritonitis was more than after half a day, 1.9 times (p<0.01) in both terms, and after 3 days it didn’t change (p˃0.05), which reflects the progression of damage to endothelium of blood vessels by day 1 of EP. The absence of subsequent dynamics in the amount of CECs by day 3 may be a consequence of an active inflammatory process and insufficiency of the system of mononuclear phagocytes which eliminate CECs. Thus, during all the studied periods of EP, an increase in the content of MDA and decrease in the concentration of GSH were observed in BP and PF of rats, indicating the development of oxidative stress, and an increase in the number of circulating endothelial cells in blood, which corresponded to a unidirectional change in the level of NOx and could indicate a relationship between activation of inducible NOS, changes in prooxidant-antioxidant state and damage to endothelium of blood vessels.
Figure 2 Section of the anterior abdominal wall (A, C) and ileum wall (B, D) in rats of control group without inflammatory and destructive changes (A, B), in rats with peritonitis with signs of severe leukocyte infiltration and mesothelium desquamation (C, D) in 3 days after peritonitis modeling. The arrows show the mesothelium. Staining with hematoxylin and eosin. Digital micrograph. Magnification x 400 (A, C), x 1000 (B, D).
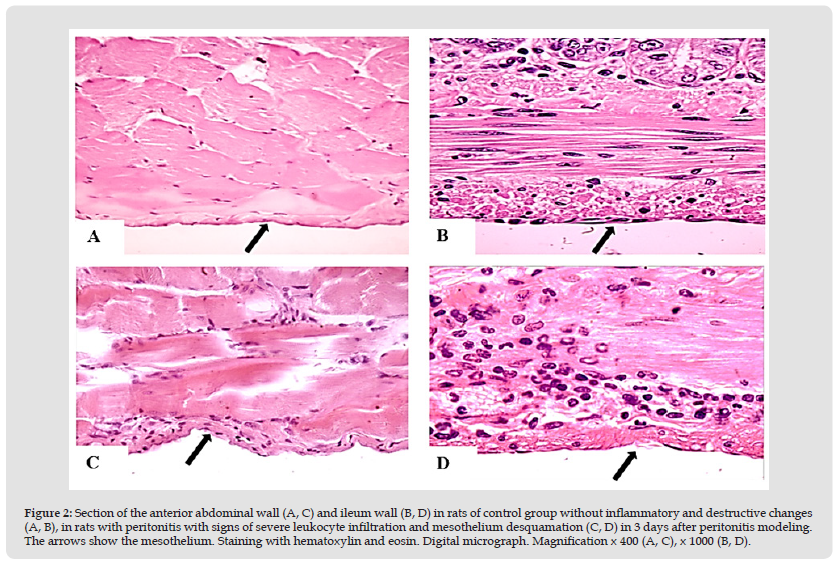
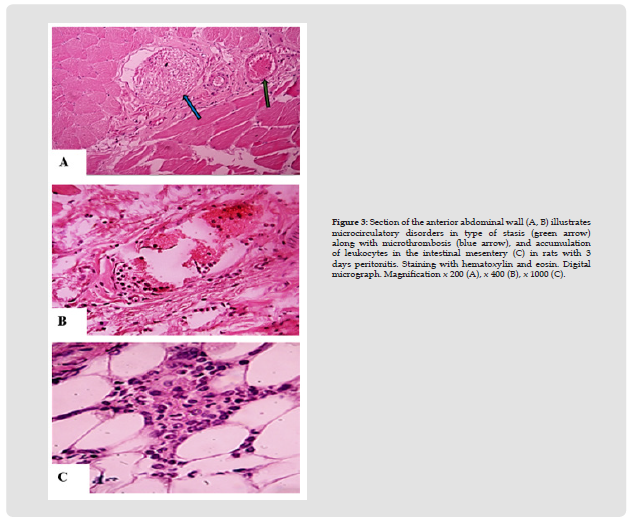
Under the conditions of EP modeling, changes in the structure of peritoneum were noted, compared with the signs in rats of the control group. At the same time, after half a day of inflammation, macroscopically marked hyperemia with single petechiae (+++), deposition of fibrin on the peritoneum surface, the presence of slightly turbid exudate in abdominal cavity (++), expansion of intestinal loops were found (Figures 2 & 3). Microscopy revealed swelling and local desquamation of mesotheliocytes (++), edema and local fragmentation of connective tissue fibers (++) with their moderate or severe neutrophilic infiltration, detection of single micro abscesses (++/+++), with signs of moderate or severe venous congestion and stasis (++), along with a slight swelling of myocytes and neurons of the intermuscular nerve plexus of the ileum. After 3 days of EP, morphological changes in the peritoneum were more pronounced than after half a day, which was indicated by change in nature of the deposits on the peritoneum to purulent-fibrinous, an increase in the turbidity of the exudate (+++), and on microscopy, the severity of desquamation of mesothelial cells (+++ ), fragmentation (+++) and leukocyte infiltration of the connective tissue fibers of the peritoneum (+++), microcirculatory disorders in the form of venous hyperemia and stasis with single microthromboses (+++), the appearance of loose adhesions, a pronounced accumulation of leukocytes in the intestinal mesentery, as well as swelling of myocytes and neurons of the intermuscular nerve plexus, sometimes with signs of destruction (+++).
Thus, inflammation of visceral and parietal layers of the peritoneum served as the morphological substrate of acute EP, which was manifested by a violation of its structure, leukocyte infiltration and changes in microcirculation.
Figure 3 Section of the anterior abdominal wall (A, B) illustrates microcirculatory disorders in type of stasis (green arrow) along with microthrombosis (blue arrow), and accumulation of leukocytes in the intestinal mesentery (C) in rats with 3 days peritonitis. Staining with hematoxylin and eosin. Digital micrograph. Magnification x 200 (A), x 400 (B), x 1000 (C).
The study of the development of experimental peritonitis in rats, modeled by intraperitoneal administration of fecal suspension, revealed:
1. An increase in the content of nitrite/nitrates, indicating an increase in NO production, as well as an increase in the concentration of the lipid peroxidation product and a decrease in the level of antioxidant protection in blood plasma and peritoneal fluid, characterizing the development of oxidative stress;
2. Pronounced desquamation of endotheliocytes of blood vessels, indicating the development of endothelial dysfunction;
3. Significant violation of the peritoneum structure;
4. Change in the studied parameters in all periods of experimental peritonitis to a greater extent in the peritoneum and peritoneal fluid than in blood, which reflects the development of inflammatory process at the local level.


