Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Georgiadis Theofylaktos1, Kourti Areti1, Thisiadou Katerina1, Tsakiridis Ioannis4, Georgiou Elisavet2, Theodorakis Dimitrios3, Parastatidis Dimitrios3, Gountenoudi Vasiliki Olympia3, Georgiadis Ioannis1, Iliadis Stavros2 and Makedou Kali1*
Received: June 14, 2023; Published: June 21, 2023
*Corresponding author: Kali Makedou, Laboratory of Biochemistry, AHEPA University Hospital, School of Medicine, Aristotle University of Thessaloniki, Greece
DOI: 10.26717/BJSTR.2023.51.008059
Background: Vitamin D is a hormone produced in the skin by ultraviolet radiation and plays a crucial role
in calcium homeostasis. It has been lately acknowledged that vitamin D participates in the pathophysiology
of various chronic diseases and its deficiency has been linked to the deterioration of patients’ condition.
The aim of this study was to record levels of 25(OH)D in the population of a rural, near-border area of
Northern Greece and to investigate possible association to an underline chronic disease. These are the
preliminary results of a study on vitamin D deficiency epidemiology and genetic polymorphisms of its
receptor and transfer protein.
Materials and Methods: 293 subjects, 111 (38%) men and 182 (62%) women, of mean age ± SD 57.6 ± 17
years, were recruited. Blood was drawn and 25(OH)vitamin D [25(OH)D] levels were determined in serum.
Statistical analyses were performed by SPSS v.25.0.
Results: In total, 149 (51%) subjects had insufficiency and 61 (20.9%) deficiency of vitamin D. 108 (37%)
were overweight and 113 (38.7%) were obese and multiple logistic regression analysis showed that
vitamin D insufficiency was significantly associated with BMI (OR: 1.049; 95% CI: 1.002-1.099; p=0.043).
Personal history revealed that 42 (14.4%) had diabetes mellitus, 12 (4.1%) had cardiovascular diseases,
12 (4.1%) had an autoimmune disease, and 153 (52.4%) had cancer or another disease. 99 (33.9%) of the
participants had received vitamin D. In the subgroup analysis, for those not taking vitamin D, insufficiency
was significantly associated with age (OR: 1.030; 95% CI: 1.009-1.053; p=0.006) and deficiency with BMI
(OR: 1.049; 95% CI: 1.002-1.098; p=0.04), whereas for those having been supplemented with vitamin D,
insufficiency was associated with obesity (OR: 4.097; 1.527-10.996; p=0.005).
Conclusions: Almost three quarters of the subjects presented with insufficiency or deficiency of vitamin
D in this area of Northern Greece. Vitamin D levels were mainly associated with age and BMI and less with
co-existing pathologies.
Keywords: Vitamin D; Deficiency; Chronic Diseases
Abbreviations: BMI: Body Mass Index; CVD: Cardiovascular Diseases; LDL: Low-Density Lipoproteins; SLE: Systemic Lupus Erythematosus; MS: Multiple Sclerosis; IBD: Inflammatory Bowel Disease; RA: Rheumatoid Arthritis; PA: Psoriatic Arthritis; ORs: Odds Ratios
Vitamin D is a fat-soluble corticosteroid that can be either synthesized in the lower levels of epidermis of the skin from 7-dehydrocholesterol due to ultraviolet radiation (290-315nm), and is called cholecalciferol (D3), or received from plants and fungi, and is called ergocalciferol [1]. Its main role is to regulate calcium, magnesium, and phosphorus metabolism, by increasing their intestinal absorption. Nevertheless, it has been lately shown that vitamin D has many extra-skeletal actions, participating in the pathogenesis of various chronic pathological conditions, such as diabetes, cardiovascular diseases, autoimmune disease, cancer, etc [2]. After vitamin D has been synthesized or received from diet it is activated in a two-step hydroxylation process, by enzymes of the family of cytochrome P450 (CYP). Cholecalciferol is transferred to the liver, where it is converted to calcifediol [25-hydroxyvitamin D, 25(OH)D]. Bound to an a-globulin, called vitamin D binding protein (VDBP), 25(OH)D reaches the kidneys where the second hydroxylation takes place and the biologically active calcitriol [1,25-dihydroxyvitamin D, 1,25(OH)2D] is finally formed. VDBP binds 25(OH)D with 10-100 higher affinity than that of 1,25(OH)2D [3,4]. Vitamin D plays a significant role in calcium homeostasis and metabolism, increasing calcium absorption in the gut, bone absorption by osteoclasts, and calcium reabsorption in distal renal tubules. It acts like steroid hormones, binding to nuclear receptors (vitamin D receptors, VDRs) and affecting gene transcription, as well as peptide hormones, connected to cell surface receptors. It has been found in many other cells and organs not related to calcium metabolism, such as immune cells, cardiomyocytes, prostate cells, pancreas, etc. [4,5].
Vitamin D extra-skeletal actions include anti-inflammatory and immune compromising properties, a role in the development and proper function of the central nervous system, appetite reduction, regulation of the cardiovascular system, anti-carcinogenic actions, etc. Therefore, it has been acknowledged that vitamin D participates in the pathophysiology of various chronic diseases, such as diabetes, cardiovascular diseases, autoimmune diseases, and cancer, and its deficiency has been linked to the deterioration of patients’ condition. The Endocrine Society has recommended the optimum vitamin D levels to be above 30 ng/mL ideally between 40 and 60 ng/mL. Serum levels between 21 and 29 ng/mL are considered “insufficiency”, and levels lower than 20 ng/mL are “deficiency” [6]. The aim of this study was to record levels of 25(OH)D in the population of a rural, near-border area of Northern Greece and to investigate possible association to an underline chronic disease. These are the preliminary results of a study on vitamin D deficiency epidemiology and genetic polymorphisms of its receptor and transfer protein, which has never been done before in this part of Europe, as far as we know.
In the present study, 293 subjects, 111 (38%) men and 182 (62%) women, of mean age ± SD 57.6 ± 17 years, were recruited after their written informed consent was given. Subjects under vitamin D supplementation were excluded from the study. 280 (95.9%) were Greeks, 7 (2.4%) originated from Albania, 2 (0.7%) from Syria, 1 (0.3%) from Bulgaria, and 2 (0.7%) from Afghanistan. Blood was drawn and isolated serum samples were stored at -20°C. 25(OH)D levels were determined in serum, on COBAS 8000 modular analyzer (Roche Diagnostics, Mannheim, Germany) using capture chemiluminescence. Personal history and anthropometrics of all subjects were recorded. The study was conducted according to the principles of the Declaration of Helsinki and was approved by the Bioethics Committee of the School of Medicine, Aristotle University of Thessaloniki (Protocol number: 192/5-6-2020).
Continuous variables are presented as mean (SD), while the categorical variables as n (%). The independent samples t-test and one-way ANOVA were used to identify differences in serum vitamin D among different groups, while the Pearson correlation coefficient was used to identify differences between continuous variables. Additionally, using serum vitamin D value as a categorical variable with the cutoffs of 30 (insufficiency) and 20 (deficiency), we performed two multivariate logistic regression analyses (backward elimination-conditional) to evaluate possible independent predictors. Odds ratios (ORs) with 95% confidence intervals (95% CI) were estimated, while statistical significance was set at 5%. All the analyses were performed by SPSS v.25.0.
In total, 149 (51%) subjects had insufficiency and 61 (20.9%) deficiency of vitamin D. 108 (37%) were overweight and 113 (38.7%) were obese. Multiple logistic regression analysis showed that vitamin D insufficiency was significantly associated with increased body mass index (BMI) (OR: 1.049; 95% CI: 1.002-1.099; p=0.043) and there was a significant negative correlation between 25(OH)D and BMI (r=-0.147, p=0.013), but not significant correlation to age, hours of outdoor activity daily, and months of vitamin D supplementation in the past (Table 1, Figure 1). Moreover, the mean BMI of vitamin D-deficient subjects was 29.7 (SD 6.3) kg/m2, significantly higher than that of subjects without deficiency [28.8 (SD 6.1) kg/m2]. Similarly, subjects with vitamin D insufficiency had higher BMI than those with normal 25(OH)D levels (Figure 2). Personal history investigation revealed that 42 (14.4%) subjects had diabetes mellitus, 12 (4.1%) had cardiovascular diseases, 12 (4.1%) had an autoimmune disease, and 153 (52.4%) had cancer or other diseases. 99 (33.9%) of the participants had received vitamin D sometimes in the past. The results of the logistic regression analysis are shown in Table 2 and revealed which factor is significantly associated with serum levels of vitamin D. BMI seems to be a dominant factor being associated with both insufficiency and deficiency of vitamin D. Participants were also divided into two subgroups according to whether they had ever been supplemented with vitamin D or not.
Table 1: Correlation of levels of 25(OH)D with age, BMI, hours of outdoor activity daily, and months of vitamin D supplementation in the past. BMI: body mass index.
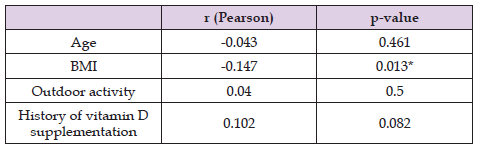
*Statistically significant.
Table 2: Factors related to vitamin D serum levels in subjects with vitamin D insufficiency and deficiency; CVD: Cardiovascular Diseases; BMI: Body Mass Index.
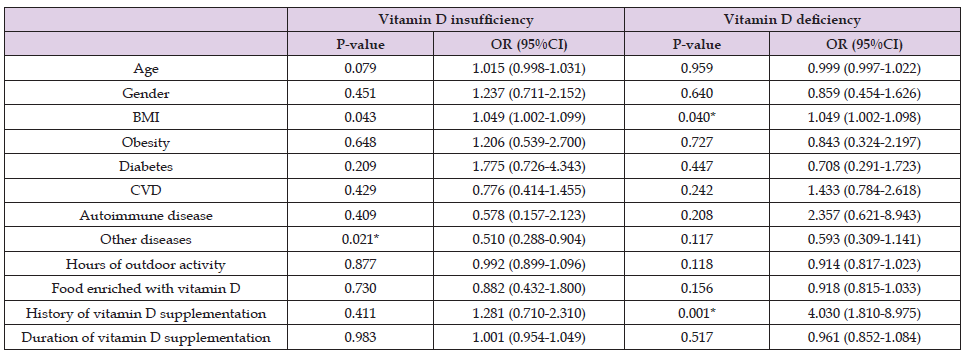
*Statistically significant.
In the subgroup analysis, for those not having received supplements of vitamin D, insufficiency was significantly associated with age (OR: 1.030; 95% CI: 1.009-1.053; p=0.006) and deficiency with BMI (OR: 1.062; 95% CI: 1.007-1.120; p=0.028), whereas for those supplemented with vitamin D in the past, insufficiency was associated with obesity (OR: 4.097; 1.527-10.996; p=0.005).
Vitamin D seems to play a crucial role in the progression of diseases, such as diabetes, cardiovascular diseases (CVD), cancer, autoimmune diseases, obesity, etc. Studies have shown that vitamin D deficiency is significantly correlated with increased BMI and visceral fat deposition [7,8], metabolic syndrome [9], hypertension, and dyslipidemia [10], which are macrovascular complications of diabetes. Vitamin D deficiency has been also found to be associated with diabetic microvascular complications, i.e., neuropathy, nephropathy, and retinopathy [11]. In specific, it has been shown that vitamin D deficiency can cause nerve damage resulting in a decrease in pain threshold [12], it can induce retinopathy by affecting the immune system and angiogenesis [13] and can induce kidney damage and proteinuria in diabetic patients [14]. Moreover, vitamin D deficiency has been shown to be related to poor glycemic control, and vitamin D supplementation is thought to act as epigenetically increasing insulin sensitivity [15,16]. In our study, levels of vitamin D did not seem to be strongly related to the presence of diabetes.
Vitamin D deficiency is assumed to be one of the early stages of metabolic disorders having an impact on CVD. In particular, hypertension due to renin-angiotensin system disorder, hyperlipidemia, especially increased low-density lipoproteins (LDL), hyperglycemia in diabetic patients and increased inflammation and oxidative stress biomarkers are some of the mechanisms probably connecting vitamin D deficiency and CVD [17]. Haybar et al. concluded that vitamin insufficiency is related to decreased coronary flow, which impairs endothelial function and promotes atherosclerosis [18]. Another study, that of Nizami et al. [19] has linked vitamin D insufficiency to myocardial hypertrophy, leading to poor cardiac muscle performance. Heart damage and myocardial infarction have also been associated with low vitamin D levels and the presence of VDRs in the lining of the blood vessels indicates that the hormone has positive actions on vascular disorders [20]. It seems that vitamin D reduces endoplasmic reticulum stress and oxidative stress, protecting endothelial cells and decreasing the risk of atherosclerosis. Our results indicate that low vitamin D levels were not significantly related to a personal history of cardiovascular diseases (Table 1).
In reference to obesity, our results revealed a significant negative correlation between levels of vitamin D and BMI. The association between vitamin D deficiency and obesity has been acknowledged by several studies [21,22] and it can be attributed mainly to the lipophilic nature of vitamin D that helps the vitamin be diluted in adipose tissue and makes it unavailable for use by other tissues. Moreover, obesity is usually combined with sedentary life and reduced sun exposure, and this could be another reason for vitamin D insufficiency or deficiency in obese adults. Other possible causes for this association might be insufficient dietary intake, renal function impairment, and fatty liver disease, common co-existing pathologies of obesity.
Vitamin D deficiency has been linked to the pathophysiology of autoimmune diseases, as it is considered to have immune-modulating properties [23-25]. Systemic lupus erythematosus (SLE), multiple sclerosis (MS), inflammatory bowel disease (IBD), rheumatoid arthritis (RA), and psoriatic arthritis (PA) are some of the autoimmune diseases that have been studied. Vitamin D deficiency has been linked to the development, activity, and severity of RA [26], and vitamin D levels showed a negative association with C-reactive protein levels. A high prevalence of vitamin D deficiency has been observed in patients with inflammatory bowel disease and has been linked to remission in patients with Crown’s disease. This can be attributed to the fact that vitamin D supports bacterial homeostasis and intestinal barrier integrity [27-29]. Moreover, in patients with SLE vitamin D levels were found significantly low in many studies presented in recent meta- analyses [30,31]. The results of the present study show that low levels of vitamin D, either insufficiency or deficiency, were not strongly associated with the presence of the autoimmune disease. The limitations of the present study are the fact that all subjects are inhabitants of a specific region of Northern Greece and were mostly Greeks. Another study, with a bigger sample size, of different regions of Northern Greece and of different ethnicities would add useful information to the results of the present study.
Vitamin D insufficiency and deficiency present with high prevalence among inhabitants of regions of Northern Greece. The different underlying pathologies do not seem to have a different impact on serum levels of vitamin D. Nevertheless, obesity seems to play a crucial role in circulating low levels of vitamin D, which seem to be lower with increasing age.
We would like to thank all those who helped technically in this study in any way, during the stratification of subjects, collection, and storage of samples, and recording of data, and especially: Pantazi Argiri, Tsoukala Theodora, Pithara Eleftheria, Chartomatsidou Chariklia, Dandaki Eleni, Chorapha Sophia, Trapezanidou Christina, Gountenoudi Valia, and Kamposou Alexandra.


