Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Aliyu AA1*, Usman MS1, Ombugadu A1, Ahmed HO1, Pam VA1, Ayuba SO1, Aimankhu OP1, Maikenti JI1, Ashigar MA1, Odey SA1, Uzoigwe NR1, Fadayomi VK1 and Abdullahi H2
Received: June 06, 2023; Published: June 14, 2023
*Corresponding author: Aliyu AA, Department of Zoology, Faculty of Science, Federal University of Lafia, PMB 146, Lafia, Nasarawa State, Nigeria
DOI: 10.26717/BJSTR.2023.51.008043
Parasitic diseases are common in the developing countries and of major health problems due to their high prevalence rate and its effect on both nutritional and immune status of the population. This study investigated the prevalence of gastrointestinal parasites (GIPs) among pregnant women attending antenatal clinics in Loko Development Area, Nasarawa Local Government Area (LGA), Nasarawa State, Nigeria between July, and September 2019. Stool samples were collected from two hundred and forty (240) pregnant women from four (4) clinics. Saline wet mount and flotation techniques were used to analyze the stool samples. An overall prevalence of 39.2% (94/240) was recorded. A total of 10 species of intestinal parasites were recorded: Ascaris lumbricoides, Hookworm, Trichuris trichiura, Giardia lamblia, Entamoeba histolytica, Strongyloides stercoralis, Schistosoma mansoni, Balantidium coli, Taenia saginata and Enterobius vermicularis with prevalence rates of 24 (25.5%), 9 (9.6%), 5 (5.3%), 21 (22.3%), 5 (5.3%), 13 (13.8%), 9 (9.6%), 1 (1.1%) and 2 (2.2%), respectively. The highest prevalence of the intestinal parasite was recorded in Ogufa Primary Health Care 56.7% (34/60), Onogbo PHC 50.0% (30/60), Loko PHC1 36.7% (22/60) while the least prevalence of 13.3% (8/60) was recorded in Loko PHC II. There was a significant difference (P < 0.001) in prevalence rate across the four clinics. GIPs prevalence across the three trimesters varied significantly (P < 0.001) in which the most infected pregnant women were those in their third trimester 58 (51.8%) followed by the second trimester 31 (38.8%) while first trimester women had the least GIPs prevalence 5 (10.4%). A significant variation (P < 0.001) was observed in GIPs prevalence across age groups in which those between ages 46 and 50 years were the most infected 90.9% (10/11). This study has established a 39.2% prevalence of gastrointestinal parasites in a community in Central Nigeria. It is recommended that further work be conducted in dry season as well as other parts of the Local Government Area so as to know the current status of GIPs among pregnant in the entire LGA.
Keywords: Gastrointestinal Parasites; Pregnant Women; Clinics; Loko Development Area
Intestinal Parasitic Infections are primarily caused by protozoans and helminths [1]. They are frequently transmitted via consumption of contaminated food and water and spread from person to person through fecal and oral contacts. This owes to the fact that they are prevalent in areas where there is overcrowding, limited access to clean water and poor personal hygiene [2,3]. Several species of intestinal parasites can infect humans. However, the commonly occurring intestinal parasites are Entamoeba histolytica, Giardia intestinalis, Cryptosporidium species, Isospora belli, Ascaris lumbricoides, hookworm infections, Strongyloides stercoralis, Trichuris trichiura, Enterobius vermiscularis [4,5]. Pregnant women, especially those in Africa are at greater risk of intestinal parasitic infections [6]. A recent study in Ethiopia found 553 out of 783 (70.6%) pregnant women to be infected with intestinal parasites with helminths being the predominant species [7]. In Nigeria, it was observed that 73 out of 401 (18.2%) pregnant women were living with intestinal parasite infections [8]. Intestinal parasites such as hookworms are known to cause anemia in pregnant women [9]. They contribute to adverse pregnancy outcomes such as low birth weight and impaired milk production. The roundworm, Ascaris lumbricoides infection has also been associated with diminished food intake and weight loss in pregnant women [3]. It has also been reported to affect survival, growth and cognitive performance of children born to infected mothers [10]. There is scarcity of information on the prevalence of gastrointestinal parasites among pregnant women in rural areas. Hence, this study investigated the prevalence of gastrointestinal parasites (GIPs) among pregnant women attending antenatal clinics in Loko Development Area, Nasarawa Local Government Area, Nasarawa State, Nigeria.
Study Area
The study was carried out in Loko Development Area in Nasarawa Local Government Area of Nasarawa State, Nigeria. It has a land mass of 1.255.5 km2 with a total human population of 140, 400 people [11]. Loko is a town in Nasarawa Local Government Area of Nasarawa State in the middle belt region or north central zone of Nigeria. It is found along the river Benue bank. The town is a mini port, along the river Benue, for the conveyance of export materials, to the eastern and western parts of Nigeria. The main activities engaged by the inhabitants of Loko town are farming and fishing. Loko Development Area is made up of Bakono, Guto, Aisa, Ayele/Iggah and Loko districts.
Determination of the Sample Size
The sample size was determined using the equation by Samukaddam and Garad [12].
n = Znpq/d2
14.3% prevalence of intestinal parasites infection among pregnant women in Ghana was adopted [13].
Where:
n = Minimum sample size
z = Percentage point of standard normal distribution curve, where curve defines 95% confidence interval as 1.96 (constant)
P = Prevalence from previous study = 14.3%
q = 1-p
d = Maximum sampling error allowed at 95% confidence limit that is 0.05
n = (1.96)2 (0.143) (0.9)/ (0.05)2
= 198
Sample Collection
The fecal samples were collected from 240 pregnant women, 60 pregnant women from each selected Primary Health Care Clinics. Prior to collection of samples, written consent of the State Ministry of Health, Medical director, Husbands, and the pregnant women were obtained after being briefed on the importance of the study. A sample bottle, spoon, newspaper, and polythene paper were provided to each of the participant (pregnant women). After 10 - 15 minutes the sample was collected by the nurse in charge of antenatal. The fecal samples collected were preserved in 10% formalin and transported to the Zoology Laboratory in the Department of Zoology Federal University of Lafia for parasitological analysis.
Sample Processing
The fecal samples were processed using floatation and saline wet mount techniques.
Flotation Technique: About 10-20 g of fecal sample was crushed and mixed with 10-12 ml of saline. The mixture was filtered through two layers of damped surgical gauze into 15 ml conical centrifuge tube. The suspension was centrifuged at 1500 rpm for five minutes, the supernatant was discarded.The sediment was mixed thoroughly with 12 ml of zinc sulphate solution and was centrifuged for one minute at 2500 rpm. The tube was placed vertically in a rack and enough zinc sulphate was slowly added to the brim and the brim was covered with a glass slide. The tube was allowed undisturbed for 10 - 15 mins. The glass slide was carefully lifted, mounted, and thoroughly examined microscopically [14].
Saline Wet Mount: About 1 g of each sample was collected using a clean spatula and was inserted into a petri-dish. Two (2) ml of normal saline solution was added to the sample which was then sieved into a 250 ml beaker. The solution was collected using a pipette, which was placed on a clean grease free glass slide, a drop of lugose iodine was added to the solution for clarity, and a cover slip was used to cover the prepared sample. The sample was then placed on the stage of the microscope, which was viewed using x10 objective lens for the detection of helminth parasites [14].
Statistical Analysis
Data obtained were analyzed using R Console software (version 4.1.1) and Statistical Package for Social Sciences (version 25, IBM SPSS Incoop., IL, Chicago, USA). Descriptive statistics are presented in frequencies and percentages. Pearson’s Chi-square test was used to test for association between categorical variables. Level of significance was set at P < 0.05.
Composition of Gastrointestinal Parasites in Pregnant Women Attending Antenatal Care Clinics
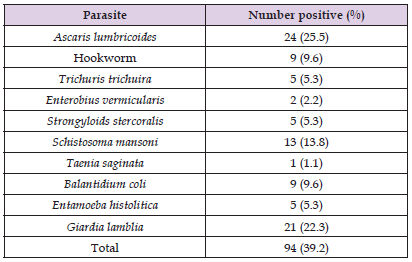
A total of 240 fecal samples were collected, 10 species of parasites were identified during the study, 5 nematodes, 3 protozoans, 1 trematode and a cestode (Table 1). Ascaris lumbricoides had the highest prevalence 24 (25.5%), followed by Giardia lamblia 21 (22.3%), Schistosoma mansoni 13 (13.8%), Hookworm and Balantidium coli 9 (9.6%) each. Trichuris trichuira, Entamoeba histolytica and Strongyloides stercoralis 5 (5.3%) each, Enterobius vermicularis 2 (2.2%) and Taenia saginata had the least prevalence with 1 (1.1%) as shown in (Table 1).
Table 1: Checklist of gastrointestinal parasites present in pregnant women attending antenatal care clinics in Loko Development Area of Nasarawa State.
Prevalence of Gastrointestinal Parasites in Relation to Clinics
Of the 240 samples examined, 94 pregnant women representing 39.2% of the study population were found to be infected with gastrointestinal parasites in Loko Development Area of Nasarawa State. Ogufa PHC had the highest prevalence of 56.7% (34/60), followed by Onogbo 50.0% (30/60), Loko PHC I 36.7% (22/60), while Loko PHC II had the least prevalence of 13.3% (8/60) as shown in (Table 2). Thus, there was a very high significant difference (χ2 = 28.08, df = 3, P < 0.001) in the prevalence of parasites in pregnant women in relation to the four clinics screened.
Note: *Significant
Prevalence of Gastrointestinal Parasites among Pregnant Women in Relation to Trimesters
A total of 48 pregnant women were examined in First trimester, 80 for Second trimester and 112 for third trimesters. Prevalence studies based on trimesters showed that pregnant women in third trimesters had the highest prevalence 61 (51.8%) followed by Second trimester 30 (38.8%) whereas First trimester was the least infected 5 (10.4%) as shown in (Table 3). Therefore, gastrointestinal parasites among pregnant women in relation to trimesters varied significantly (χ2 = 26.629, df = 2, P < 0.001).
Note: *Significant
Prevalence of Gastrointestinal Parasites in Relation to age Groups of Pregnant Women
Age specific prevalence showed that pregnant women from ages 46-50 years had the highest prevalence 10 (90.9%) followed by age group 41-45 13 (72.2%) then age group 31-35 17 (54.8%), age group 36-40 22 (50.0%), age group 26-30 19 (36.5%), age group 16-20 11 (19.3%) while age group 21-25 had the least prevalence 2 (7.4%) as shown in (Table 4). Therefore, there was a significant difference (χ2 = 107.34, df = 6, P < 0.001) in GIPs prevalence in relation to age groups of pregnant women.
Note: *Significant
Findings from this study clearly shows that pregnant women are easily susceptible to gastrointestinal parasites (GIPs) which may possibly compromise their overall well-being and that of the unborn child. Also, the occurrence of GIPs in the study area could be due to favorable environmental conditions which supports their survival, poor sanitation, and contaminated water sources for household use. The high prevalence in this study is in line with the 70.6% prevalence obtained in Ethiopia [7], 49.6% in Ghana by Tay, et al. [10], 73.9% in Venezuela as opined by Rodríguez-Morales, et al. [15], and Ayuba, et al. [16] observed a 79.5% GIPs prevalence in Karu, Nasarawa State. The higher prevalence from our study was higher in comparison to the findings by Wakesa, et al. [3], Adedoja, et al. [17] and Frederick, et al. [18] with 21% in Kenya, 33.3% in middle belt Nigeria and 2.2% in Benin City, respectively. Furthermore, significantly lower infection rate of 3.73% was reported in Iran [19]. Ascaris lumbricoides had the highest prevalence of intestinal parasites among pregnant women encountered in the study representing (25.5%). This finding is in tandem with the findings of 32.7% in Ethiopia [7] and 63.24% by Njoku, et al. [20] in Ebonyi State Nigeria where Ascaris lumbricoides is the most prevalent. This could be attributed to the ability of a single worm to release up to 200,000 eggs per day coupled with the resistant nature of the eggs which have protective proteineous coat to extreme environmental conditions. The prevalence of A. lumbricoides was followed by Giardia lamblia (22.3%). The high prevalence of these two parasites poses a serious health threat to the infected pregnant women.
These two parasites contribute to adverse pregnancy outcomes such as low birth weight and impaired milk production. Ascaris lumbricoides infection has also been associated with diminished food intake and weight loss in pregnant women [3]. It has also been reported to affect survival, growth and cognitive performance of children born to infected mothers [10]. Schistosoma mansoni accounted for 13.8% of the infections among the pregnant women. This finding is lower than 22.70% obtained by Tonga, et al. [21] in Cameroon but like previous works by Akimbo, et al. [8] in Edo, Benin city Nigeria. Hookworm and Balantidium coli contributed 9.6% of the infections each. This could be because of fecal pollution, soil, and domestic water supply around homes due to poor sanitation and improper sewage disposal. This is higher than 3.9% reported by Wekesa, et al. [3], but in consonance with Akimbo, et al. [8], Fuseini, et al. [22], and Umeh, et al. [23]. Trichuris trichuria, Entamoeba histolytica and Strogyloides stercoralis accounted for 5.3% of the infection. Other parasites observed were: Enterobium vermicularis and Taenia saginata with each of them contributing 2.2% and 1.1%, respectively. The prevalence was higher in Ogufa PHC. This outcome might be due to improper management of organic refuse and inadequate supply of clean water. Poor drainage and use of dumping sites for defecation might have contributed to this high prevalence.
Generally, there was improper management of toilet facilities in some of these areas and some do not even have one. Unavailability of portable water and farming activities (over exploitation) might derive pregnant women into unhygienic sources, there by exposing them to risk factors. The stage of pregnancy/gestational age was found to be associated with intestinal parasitic infections among women in Loko Development Area. The third trimester had the highest prevalence (51.8%), followed by second trimester (38.8%) while the first trimester was the least prevalent (10.4%). This agrees with the findings of Akimbo, et al. [8] and Umeh, et al. [23]. This outcome might be due to the high level of nausea and frequent vomiting which is associated with the first trimester this in turn hinders them from eating, thereby reducing the risk factors. The high prevalence in the third trimester might be due to the frequent eating of different kinds of food irrespective of the source. The infection in the pregnant individuals were all asymptomatic, this led to the accumulation of the parasite even before conception. The observed variation in GIPs prevalence in relation to age groups possibly suggests that women aged 46 to 50 years have a high compromised immune system thereby making them most susceptible to GIPs infections. On the contrary, Abaka-Yawson [13] reported high GIPs prevalence among younger ages.
This study shows that gastrointestinal parasites prevalence among pregnant women in in Loko Development Area in Nasarawa LGA of Nasarawa State, Nigeria was 39.2%. GIPs prevalence in relation to clinics, trimisters as well as age groups varied significantly. Poor sanitation, inadequate portable water supply, low level of personal hygiene education and lack of de-worming were observed as the major risk factors that enhances the transmission of the parasites. To this end, we hereby recommended that the Primary Health Care service unit of Nasarawa LGA should embark on public health education in order to improve the level of hygiene in the area; the State Government should provide potable drinking water to the rural areas such as Loko Development Area; and a large scale monthly or quarterly de-worming exercise should be carried out in all the PHCs for intending pregnant women since antiparasitic drugs has its side effects on pregnancy.


