Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Daniela Tirotta*, Claudia Lena and Paolo Muratori
Received: June 05, 2023; Published: June 13, 2023
*Corresponding author: Daniela Tirotta, Internal Medicine, Forlì, Morgagni Pierantoni Hospital, ausl Romagna, Italy
DOI: 10.26717/BJSTR.2023.51.008041
Given the invasiveness of liver biopsy, new non-invasive methods to evaluate the prognosis of autoimmune liver diseases continue to be searched. We introduce a case report and a review of cases of autoimmune hepatitis (AIH) and primary biliary cholangitis (CBP) associated to halo sign and inhomogeneity of the liver parenchyma on diagnostic imaging (computed tomography, magnetic resonance imaging and contrastenhanced ultrasound). Our clinical case is a 37-year-old patient came to our Unit for an autoimmune hepatitis, characterized by atypical imaging: abdomen ultrasound showed hepatomegaly with pseudonodular areas and contrast-enhanced ultrasound (CEUS) manifested both images of acute liver disease (periportal edema) and chronic liver disease (initial irregularity of the liver profile, without hypertrophy of the left hepatic lobe and caudate) Abdominal computed tomography (CT) and magnetic resonance imaging (MRI) confirmed an epatomegaly with inhomogeneous enhancement in the right lobe portal phase and periportal edema (halo sign). Liver biopsy showed intense lymphocytic, plasma cell and neutrophilic infiltrates with aeosinophilic granulocytes in the portal, periportal and lobular sites and large areas of hepatocyte necrosis. Six months later of theraphy (steroid and azatioprine) the patient was asymptomatic, but she had persistent liver inhomogeneity, therefore she performed a fibroscan. We conducted a sensitive review of the literature: periportal cuffing on RMI and hepatic halo sign in CT and MRI are described in various liver diseases and in some cases it seems to be a prognostic indicator, including in autoimmune disease. We describe the clinical and instrumental aspects of these cases. In literature there are no descriptions of the ceus pattern in this setting, which could be a sensitive helpful imaging method to discriminate prognosis and correlation with staging/grading of autoimmune liver diseases.
Keywords: Autoimmune Liver Diseases; Autoimmune Hepatitis; Primary Biliary Cholangitis; Halo Sign; CEUS; Abdominal CT; Abdominal RMI
Abbreviations: AIH: Autoimmune Hepatitis; CBP: Primary Biliary Cholangitis; CEUS: Contrast-Enhanced Ultrasound; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; TE: Transient Elastography; DWI: Diffusion-Weighted MRI
The most frequent autoimmune liver diseases are autoimmune hepatitis (AIH) and primary biliary cholangitis (PBC). The application of published criteria of AIH allows a ready diagnosis, but often the onset of the disease is insidious and progression is gradual without symptoms. Therefore, the diagnostic work-up is based on non-organ specific autoantibodies associated with polyclonal hypergammaglobulinemia and typical or compatible histology in the absence of viral hepatitis markers. In particular histology is essential in the diagnosis [1-2]. Instead the diagnosis of PBC is usually based on combination of clinical findings, cholestatic biochemical pattern, and the presence of specific antibodies. Liver biopsy is not routinely required except in antibody negative patients, but the biopsy can give information on necroinflammatory activity and grading of liver fibrosis [3,4]. In both cases, due to limitations of liver biopsy, new non-invasive tests such as, transient elastography (TE) and abdomen magnetic resonance (abdomen MRI), might be of helpful to detect the assessment of liver fibrosis and could provide additional information about disease progression and treatment response. In particular transient elastography produce immediate evaluation of extension of liver fibrosis greater than that of the biopsy. However, it has several limitations, as the accuracy in the presence of ascites and obesity, the poor availability and the impossibility of multisegmental analysis. Two types of MRI are used in cholestatic disease: Diffusion-weighted MRI (DWI) (based on altered diffusion of water protons in fibrotic tissue), which provides insight into liver fibrosis distribution, with high sensitivity and specificity, and MRI with conventional sequences, used in the follow-up of the disease, especially for complications [5]. Histopathologically in both CBP and AIH, inflammation initially affects the portal space. Imaging methods can identify two characteristic findings in this space: the periportal hiperintensity (cuffing) on T2- weighted MRI and the halo sign on abdominal CT and MRI.
Periportal edema, inflammatory cell infiltration, ductular proliferation and dilatation of lymph vessels in the portal triads, seen as T2-weighted periportal hyperintensity (cuffing), is suggestive for persistent inflammation in the periportal spaces. This finding can be associated with the fluid or dilated lymphatics: probably in patients with severe hepatitis (as in congestive heart failure, or microvenous occlusive disease) the liver may become congested and edematous, leading to increased production of lymph. The increased volume of lymph fluid cause, by compression, a secondary distension of lymphatic vessels and edema in the periportal spaces (5) Halo sign is a circumferential zone of decreased attenuation around the peripheral or subsegmental portal venous branches in abdomen CT (5) or RMI (6), which has no an exact pathophysiologic basis, but it might be due to a periportal hepatocellular parenchymal extinction associated to rosette of large regenerating nodules. These findings indeed is more widespread in the patients with imaging periportal halo sign than those without. The “periportal halo sign” in MRI, described as a rounded lesion area of hypointensity both T1- and T2- weighted MR, of 5mm-1cm, centered on the portal vein, is suggestive for deposition of fibrous tissue and extinction of hepatocellular parenchyma around the portal triads, more commonly in advanced disease. However, due to the irregular distribution of liver fibrosis, both periportal hyperintensities and periportal halo can be observed in the same patient [5,6]. Anyway in PBC the incidence of cuffing is 100% in the early stages and 33% in the advanced stage (13). We described our case of AIH associated to diffuse inhomogeneity of the liver parenchima and to periportal edema on CEUS and RMI and we performed a sensitive serch of the literature on the cases in which periportal edema is predictive for the prognosis of CBP or AIH. We have not found reports concerning the application of CEUS in this setting.
We introduce a case report and a review of cases of HAI and CBP associated to halo sign and inhomogeneity of the liver parenchyma published in the literature. We performed a sensible research on PubMed (Keywords “autoimmune hepatitis’’ and ‘’primary biliary cholangitis” and “MRI” and “CT” ‘’Contrast-enhanced ultrasound“ AND “halo sign”, Mesh term “autoimmune hepatitis” and “RMI” “primary biliary cholangitis” and “RMI”), with control of references. We obtained 9 cases (6-14); age limit (adult: 19+ years) and language limit (English language) (Table 1).
Clinical Presentation
Our Case
A 37-year-old patient is hospitalized due to abdominal pain associated with nausea, asthenia, jaundice. Her medical history showed: hypertension after the last pregnancy; previous Sars Cov-2 infection (5-6 months before ), recent course of antibiotic therapy with fosfomycin (2 days), then ciprofloxacin (3 days). Tests showed normal white blood cells and inflammation indexes; hepatic necrosis and cholestasis (total bilirubin 4.49 mg/dl: direct 3.9 mg/dl, ALT/ AST 1478/870 U/L; alkaline phosphatase 161 U/L, gamma GT 133 U/L). Our abdomen ultrasound showed hepatomegaly and some pseudonodular areas and manifested images suggestive both for acute liver disease (periportal edema) and for chronic liver disease (initial irregularity of the liver profile, without hypertrophy of the left hepatic and caudate lobe). We hypothesized: a vascular etiology, as Budd Chiari syndrome (areas of parenchymal inhomogeneity, perivascular edema); an autoimmune hepatitis (acute onset, with asthenia, however large inhomogeneity of the parenchyma, no autoimmune comorbidity); a DILI (recent antibiotic therapy, but quinolones and phosphomycin not typically associated with DILI); a viral hepatitis (Halo sign , acute onset); a IgG4-related hepatitis (however localized only to the liver). Tests showed negativity of viral tests (HCV, HCV, HAV, HEV, CMV, EBV), ANA 1:80, nucleolar pattern, AMA, ASMA, LKM antiphospholipids negatives, IgG4 and Immunoglobulins normal. Also pre/post sinusoidal vein thrombosis was absent.
Literature Review
The cases selected from the literature are all radiological cases, therefore a description of the clinical presentation of the patients is not reported.
Our Case
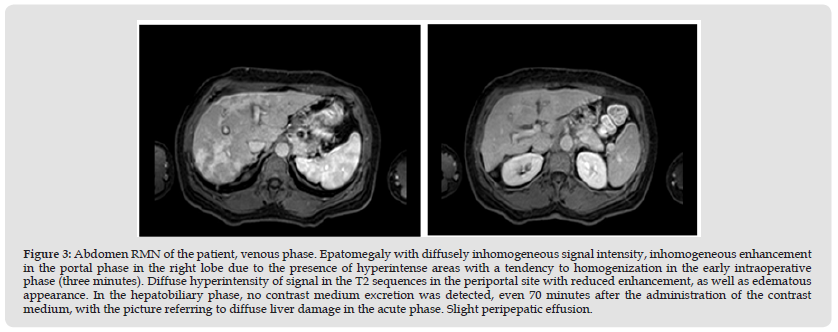
Abdominal CEUS (Figure 1) showed inhomogeneous hepatomegaly. In the portal phase, especially sinusoidal, starting from 2 minutes, important reduction of the periportal wash-in was evident, as in edema/inflammation of liver parenchima. Abdominal CT (Figure 2) and MRI (Figure 3) confirmed an epatomegaly with inhomogeneous enhancement in the right lobe portal phase, periportal edema (halo sign). Liver biopsy showed intense lymphocytic, plasma cell and neutrophilic infiltrates with aeosinophilic granulocytes in the portal, periportal and lobular sites.
Figure 1 Ceus of the patient. Inhomogeneous hepatomegaly. In the portal phase, especially sinusoidal, starting from 2 minutes, important reduction of the periportal wash-in, as in edema/inflammation of liver parenchima was evident.

Figure 2 Abdomen CT of the patient. Liver within the volumetric limits, in the precontrastographic phase relatively hypodense patches are observed as from patchy steatosis. The liver profiles appear somewhat irregulars, however, without hypertrophy of the left hepatic lobe and the caudate. In the portal phase, a marked and irregular patchy enhancement is observed especially in the right hepatic lobe, which tends to isodensity in the late phase. Greater representation of the periportal spaces is observed as per edema, no signs of mass effect on the portal structures are observable. Presence of a 30 x 11 mm hilar lymph node. Peritoneal effusion in the Douglas fissure.
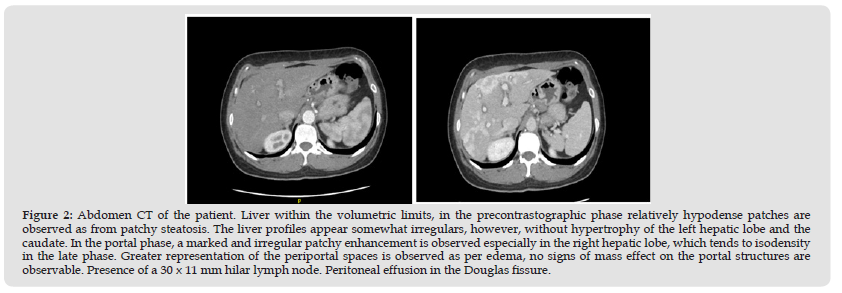
Figure 3 Abdomen RMN of the patient, venous phase. Epatomegaly with diffusely inhomogeneous signal intensity, inhomogeneous enhancement in the portal phase in the right lobe due to the presence of hyperintense areas with a tendency to homogenization in the early intraoperative phase (three minutes). Diffuse hyperintensity of signal in the T2 sequences in the periportal site with reduced enhancement, as well as edematous appearance. In the hepatobiliary phase, no contrast medium excretion was detected, even 70 minutes after the administration of the contrast medium, with the picture referring to diffuse liver damage in the acute phase. Slight peripepatic effusion.
Literature Review
In literature in CBP the periportal hyperintensity on T2-weighted MRI seems to reflect an active inflammation in the portal tracts and periportal hyperintensity was seen even in the late stage of PBC, but it is more frequent in the early stages. It seems that the grading of the periportal halo sign is correlated with the histological stage of fibrosis. In one case [7-13], periportal edema was also described on CT. Only one study investigates the periportal aspect on MRI in autoimmune hepatitis [14] and enlarged periportal space seems to have a significant positive correlations with fibrotic stage.
Our Case
Revised Original Scroring system of the patient was 11. We hypothesized an autoimmune hepatitis and we started steroid and then steroid and azathioprine therapy. Six months later the patient was asymptomatic, liver function tests normalized, but she had persistent liver inhomogeneity in US bmode. We performed a fibroscan (F0-F1 according to CBP-CSP). Therefore, the normalization of the CEUS picture preceded the laboratory one, even if signs of hepatic inhomogeneity persisted in ultrasound.
Literature Review
The cases selected from the literature are all radiological, therefore a description of the clinical evolution of the patients is not reported, but a positive correlation is described between the halo sign and the degree of liver fibrosis.
Identifying a non-invasive methods, that can predict the grading and the staging of the autoimmune liver disease could be of great helpful, to improve the management of these patients. Since liver inflammation in both CBP and AIH begins in the periportal space, the early and non invasive detection of periportal edema/fibrosis could be a reliable prognostic marker. Indeed imaging cannot replace liver biopsy especially in autoimmune hepatitis, where it remains essential for the search for lymphoplasmacytic infiltrate in interface hepatitis, but it can be helpful in establishing degree of activity and stage in hepatic autoimmune disease. Our review of cases would lead to the conclusion that T2 hyperintensity on MRI (cuffing) correlates to the activity of inflammation of CBP, as CEUS in our case, while the halo sign to the diagnosis of CBP and to the fibrotic evolution. Actually there are few related studies, which have several limitations: studies are are all observational, retrospectives, or case series, these studies are radiological, non-clinical; almost all involve CBP, only one case an AIH; almost all concern the MRI and in a few cases CT. Therefore, large prospective sare necessaries, including cases of AIH as well as CBP and which also make use of CT, would be necessary. Finally, since there are no studies in the literature that analyze the ceus aspect of the periportal space in autoimmune liver diseases, and since ceus is a real time and less expensive diagnostic method, not associated with radiation, studies concerning the prognostic aspect of ceus images on liver autoimmune disease and their correlation with MRI would be appropriate.


