Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Filip Pistulli1, Ergys Ramasaço2, Albert Koja3 and Edmond Pistulli4*
Received: May 29, 2023; Published: June 12, 2023
*Corresponding author: Edmond Pistulli, Pediatrician Professor at Tirana University of Medicine, Albania
DOI: 10.26717/BJSTR.2023.51.008034
Abbreviations: BP: Blood Pressure; TG: Triglycerides; WC: Waist Circumference; NAFLD: Non-Alcoholic Fatty Liver Disease; NASH: Nonalcoholic Steatohepatitis; GDM: Gestational Diabetes Mellitus; OSA: Obstructive Sleep Apnea; WHO: World Health Organization; BMI: Body Mass Index; IR: Insulin Resistance; WMD: Weighted Mean Difference; HSCRP: High-Sensitivity C-Reactive Protein; LDL: Low-Density Lipoprotein; ALT: Alanine Aminotransferase; ADA: American Diabetes Association; OGTT: Oral Glucose Tolerance Test; AAP: American Academy Of Pediatrics; CVD: Cardiovascular Disease
Metabolic syndrome is defined by a constellation of physiological, biochemical, clinical, and metabolic factors [1]. The metabolic syndrome (MetS) is defined also as the presence of at least three of the following five common clinical measures, which occur in people with insulin resistance: elevated triglycerides (TG), low high-density lipoprotein cholesterol (HDL-C), elevated blood sugar, elevated blood pressure (BP) and elevated waist circumference (WC) (Lear. S.A, et al. [2]). MetS is a term that serves as an umbrella of risk factors for individuals to be at an increased risk of disease [3]. This disease is also known by other names such as: Syndrome X, Insulin Resistance Syndrome and Dismetabolic Syndrome [4]. MetS predicts cardiovascular disease and type 2 diabetes [5]. The metabolic syndrome is most widely used for describing the metabolically associated disorders including obesity, insulin resistance, type 2 diabetes, hypertension, dyslipidemia, cardiovascular disease, non-alcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis (NASH), and cholesterol gallstone disease [6]. Focusing attention on children with cardio metabolic risk factors is emphasized on the need to determine a “ child-specific metabolic syndrome”[7]. According (WHO), to the Joint Task Force, adults must complete 3 of the following 5 criteria for metabolic syndrome: abdominal perimeter above defined values, glycemia > 100mg / dl, triglycerides > 150 mg / dl [1], systolic pressure > 130 mmHg and diastolic pressure > 85 mmHg or untreated hypertension [8], HDL <40 mg / dl in men and HDL 50 <mg / dl in women [9]. These individual components of MetS occur together more often than would be expected by chance—as though they are driven by similar underlying processes that lead to insulin resistance, including cellular dysfunction in adipocytes, myocytes, and hepatocytes; oxidative stress; and cellular inflammation [10] (Figures 1 & 2).
National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III ) criteria of the metabolic syndrome were defined as: at least three of the following criteria: Waist circumference >102 cm in men or >88 cm in women, plasma triglycerides ≥150 mg/dl (1.7 mmol/L), HDL cholesterol <40 mg/dl (1.03 mmol/l) in men or <50 mg/dL (1.29 mmol/l) in women, blood pressure ≥130/85 mm Hg, and fasting plasma glucose ≥110 mg/dl (6.1 mmol/l) [11].
Risk Factors in Pediatric Age
Maternal/Hereditary Factors: Duration of breastfeeding is inversely proportional to obesity in later life. Childhood metabolic syndrome is also attributed to relation with gestational diabetes mellitus (GDM) and large birth weight (Barker’s hypothesis). Thrifty phenotype hypothesis and fetal origins of adult disease are other hypothesis describes the antenatal and perinatal factors describing the impact of birth weight & features of insulin resistance syndrome in infants. There must be other hereditary factors also responsible for development of metabolic syndrome. Risk of obesity is more than double if one parent is obese. Children with at least one parent with the metabolic syndrome had significantly more obesity and insulin resistance than control [12]. Obesity in childhood is the main determinant of whole body reduced insulin sensitivity. A tight relation exists between typical lipid deposition patterns, specifically within the skeletal muscle and liver, as well as the intra-abdominal compartment and whole body insulin sensitivity. The impact of lipid deposition within insulin responsive tissues such as the liver and skeletal muscle relates to the ability of fatty acid derivates to inhibit elements of the insulin signal transduction pathway. Strengthening the relation of obesity and reduced insulin sensitivity are the observations that weight gain reduces insulin sensitivity while weight loss increases it. This manifests as the appearance of cardiovascular risk factor clustering with weight gain and its recovery in the face of weight loss. Both obesity per se, via the adipocytokine profile it induces, and low insulin sensitivity, are independent determinants of the adverse metabolic phenotype characteristic of the metabolic syndrome [13].
Lifestyle Factors: Lifestyle factors like lack of physical activity, fatty food consumption, preference for simple carbohydrates and fewer vegetables are more important factors than hereditary factors probably for development of metabolic syndrome in adolescents. Eating junk food incessantly and increased screen time in front of television and computers are the emerging risk factors for metabolic syndrome in twenty first century. Especially the children of parents with higher socioeconomic status are more reluctant to physical activity and preferring indoor games and unhealthy food habits [12]. Many studies strengthen the evidence of an association between low levels of physical activity and early precursors of metabolic syndrome [14]. In a study of 1,426 Hispanic and Latino youth, (Strizich, et al. [15]) found that lower levels of PA were associated with unfavorable glucose/lipid metabolism and increased inflammatory markers. Another study, a three year longitudinal one how belongs physical activity , by (AgostinisSobrinho, et al. [16]). demonstrated that lower cardiorespiratory fitness was associated with higher systolic blood pressure at both baseline and follow-up. Tobacco smoking which remains an important risk factor for metabolic syndrome in adults, although less prevalent in children, still its incidence is increasing in recent days in adolescents of India and other countries [12] (Figure 3).
Co-Morbid Conditions: In adults, metabolic syndrome is considered one of the most important risk factors for coronary vascular disease and type two diabetes mellitus (T2DM). Although children are at less risk of developing cardiac complications, insulin resistance is almost universally seen in these children and in later life many of them develop cardiac complications also [12]. In adults with MetS, the transition from normal glucose homeostasis to diabetes takes decades, while in youths the evolution appears to be much more rapid [17]. These children are also more susceptible to other diseases like polycystic ovary syndrome (PCOS), cholesterol gallstones, nonalcoholic fatty liver disease (NAFLD), asthma, sleep disturbances, some malignancies [12], obstructive sleep apnea (OSA), and mental health disorders [18]. Polycystic ovarian syndrome with irregular menstrual cycle and later on infertility is quite common in these children. Obstructive sleep apnea often accompanies obese children, especially those with BMI >30 kg/sqm. Severe obstructive sleep apnea and hypoventilation during sleep may lead to cor-pulmonale and right-side heart failure. In NAFLD, steatosis (excessive fat accumulation in the form of triglycerides) is found in >5% hepatocytes. It is the one of the leading causes of chronic liver disease in children [12]. NAFLD and MetS in pediatric age group,are both associated with a high risk to develop cardiovascular and diabetic complications early in life [19] (Figure 4).
Figure 4 Prevalence of metabolic syndrome in adolescent by sex and race ethnicity. Data are for adolescent participants age 12-19 years from the National Health and Nutrition Examination Survey 1999-2012 as reported in lee et al.
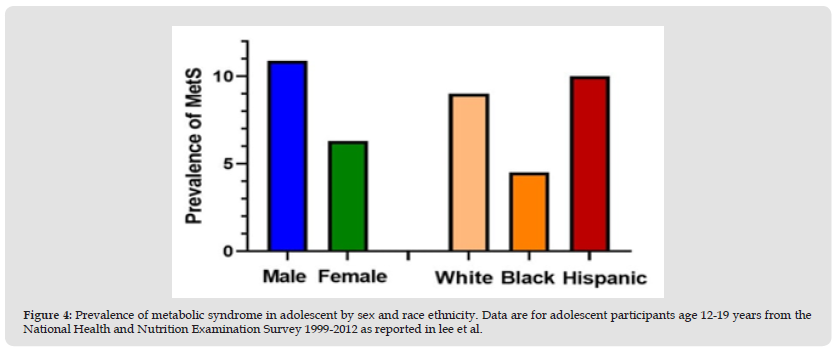
Epidemiology: It is difficult to estimate the prevalence of metabolic syndrome in children because many different criteria have been used in its multiple definitions. For non-obese, non-overweight populations, the range was 0–1%. Close to 90% of obese children and adolescents have at least one feature of the metabolic syndrome. Various publications have noticed prevalence numbers ranging from 0.2% to 38.9%. In a systematic review of 85 studies in children, the median prevalence of metabolic syndrome in whole populations was 3.3% (range 0–19.2%), in overweight children was 11.9% (range 2.8–29.3%), and in obese populations was 29.2% (range 10–66%). For non-obese, non-overweight populations, the range was 0–1% [1]. (Duncan. et al, [20]). examined trends in the prevalence of MetS in U.S. adolescents aged 12–19 yr using NHANES data (1998–1994 and 1999–2000) and the pediatric definition developed by Cook and the study showed that The MetS was most frequent in obese adolescents, with a prevalence of 32.1%, compared with only 7.1% for overweight adolescents. The underlying prevalence of MetS in adolescents depends on the set of MetS criteria used, with overall ranges in the US from 1.2%–9.8% using modified ATP-III criteria to 4.5%–8.4% using the IDF adolescent criteria.
Assessments among school-aged children and early adolescents is lower (0.2%–1.2%), which is likely because of the strong effects of puberty on insulin resistance. For example, insulin resistance as estimated by measures such as the homeostasis model of insulin resistance—which usually tracks closely with MetS—at age 8 years is half that seen among those at 15 years, consistent with the concept that puberty itself may be involved with the progression of abnormal metabolic processes [10]. In addition to variation by age, the prevalence of MetS also varies significantly by sex, with male adolescents having a greater prevalence than females (Figure). Interestingly, there is also variation by race/ethnicity, being more common in whites and Hispanics compared to African Americans (Figure) [10]. Eventually, the trend of MetS in the general population of children and adolescents in HICs was plotted in a scatter plot based on the prevalence of cases with publication year (2003 to 2020). The trend line implied that there is an increasing trend of cases in three diagnostic methods (IDF, ATP III, and de Ferranti) [21]. Among the 31 papers included in the review by Reisinger (2020), the prevalence of pediatric MetS ranged from 0.3 to 26.4% [22]. The lowest prevalence (0.3%) was found in a population-based survey of Colombian school children by using the definition of the IDF [22]. The highest prevalence (26.4%) was found among Iranian children and adolescents by using the definition of de Ferranti. et al, [23]. Prevalence was high in the Middle East (13 and 6% in Iran and almost 5% in Saudi Arabia according to the Cook definition), in the United States (5.4% by IDF and 10.1% by the (Ford. et al, [24]), and in South American countries (9.5% in Chile by IDF and 6.2% in Colombia by the Cook definition [25]. Table Re [22] Epidemiological studies investigating the prevalence of metabolic syndrome in children and adolescents based on the diagnostic criteria from the IDF, (Cook, et al. [23-25]) (Figure 5, Tables 1-4).
The pathogenic mechanisms of MetS are complex and remain to be fully elucidated. Whether the individual components of MetS represent distinct pathologies or manifestations of a common pathogenic mechanism is still debated. Of all the proposed mechanisms, insulin resistance, neurohormonal activation, and chronic inflammation appear to be the main players in the initiation, progression, and transition of MetS to CVD [26]. It has been recognized that in obesity, inflammation, with increased accumulation and inflammatory polarization of immune cells, takes place in various tissues, including adipose tissue, skeletal muscle, liver, gut, pancreatic islet, and brain, and may contribute to obesity-linked metabolic dysfunctions, leading to insulin resistance and type 2 diabetes [27]. Insulin resistance is thought to be central to the development of MetS and play a role in the pathogenesis of its individual metabolic components. The World Health Organization (WHO) hypothesizes that the association and clustering of T2D, hypertension, dyslipidemia, and CVD arises from a common antecedent - insulin resistance [2].
Patogenesis of MetS in Pediatric Age
Although the pathogenesis of metabolic syndrome is not completely understood, recent data suggest that interaction between obesity, insulin resistance and inflammation play a key-role in its development. It is suggested that accumulation of free fatty acids in the liver, adipocytes, skeletal muscles and the pancreas in the setting of obesity leads to impaired insulin signaling and subsequent insulin resistance. Insulin resistance in the liver leads to decrease in its effect on suppression of glucose production. Additionally, hyperinsulinemia causes an increase in the transcription of genes for lipogenic enzymes in the liver, which leads to increased production of triglycerides. Elevated BP in metabolic syndrome is thought to be secondary to hyperinsulinemia via mechanisms such as sympathetic nervous system activity, renal sodium retention and smooth muscle growth. Insulin has a vasodilatory effect on the endothelium secondary to the production of nitric oxide (a potent vasodilator). It is believed that inflammatory cytokines release from dysfunctional adipocytes, such as, monocyte chemoattractant protein-1, and tumor necrosis factor-alpha, promotes macrophages migration to those adipose tissues and further increase cytokine production. Additionally, a decrease in adiponectin level seen in obesity can result in more inflammatory process in the adipose tissues [1]. MetS is characterized by increased visceral as opposed to subcutaneous fat as well as ectopic fat deposited in abnormal locations, such as the liver. Ectopic fat distribution results in the release of adipocytokines, causing a state of low-grade inflammation, with increased inflammatory factors, such as plasminogen activator inhibitor-1, tumor necrosis factor α, interleukin 6, and acute phase reactants such as high-sensitivity C-reactive protein and fibrinogen. The endoplasmic reticulum acts as a nutrient sensor. Energy or nutrient excess can trigger endoplasmic reticulum stress, resulting in activation of inflammatory pathways, increased reactive oxygen species production, and mitochondrial dysfunction. Some emphasize the importance of the inflammatory state, with insulin resistance being a consequence of inflammation. Irrespective of what is the consequence or cause, insulin resistance, ectopic fat distribution, and inflammation are all key pathologic players in the components of MetS [18].
Clinical Presentation of MetS
Common conditions of metabolic syndrome area large waistline also called abdominal obesity or "having an apple shape" where the extra fat in the stomach area is a bigger risk factor for heart disease than extra fat in other parts of the body [28]. According to IDF, a participant with the MetS has a waist circumference (≥94 cm in men and ≥80 cm in women) [29]. For adults, WHO defines overweight and obesity as follows: overweight is a BMI greater than or equal to 25; and obesity is a BMI greater than or equal to 30 [30]. High blood pressure can damage the heart and blood vessels. It can also cause plaque, a waxy substance, to build up in arteries. Plaque can cause heart and blood vessel diseases such as heart attack or stroke.High blood sugar levels can damage blood vessels and raise the risk of getting blood clots. Blood clots can cause heart and blood vessel diseases. High blood triglycerides can raise the levels of LDL cholesterol, sometimes called bad cholesterol. This raises the risk of heart disease. HDL cholesterol, sometimes called good cholesterol in low levels isn’t capable enough to remove “bad” LDL cholesterol from blood vessels so “Bad” LDL cholesterol can cause plaque buildup in blood vessels [30]. Combined Dyslipidemia in adulthood, it can present with xanthomas (knees, buttocks, and elbows) and yellow-orange palmar discoloration [31]. Other well-known manifestations of MetS include polycystic ovarian syndrome in women and obstructive sleep apnea. Obstructive sleep apnea has been associated with excess body fat content, insulin resistance. Individuals with obstructive sleep apnea are at increased risk for CVD morbidity and mortality [32].
Clinical Features Seen in Metabolic Syndrome in Children are: obesity, dyslipidemia, hypertension, glucose intolerance and T2DM, nonalcoholic fatty liver disease (NAFLD), polycystic ovarian syndrome (PCOS), inflammatory markers [1].
Obesity: Is an essential component of the metabolic syndrome and the development of T2DM and CVD [33]. Obesity is diagnosed based on body mass index (BMI), with those with a BMI ≥95th percentile for gender and age are considered obese [33].
Combined Dyslipidemia: Appears as a nonspecific rash in pediatric age. In children with severe dyslipidemia can appear like xanthoma [31].
Hypertension: Is a very important component of metabolic syndrome and a modifiable risk factor for heart disease [34]. Insulin resistance is of major importance in obesity [35].
Glucose Intolerance (Impaired Fasting Glucose or Impaired Glucose Tolerance) and Diabetes Mellitus Type 2 (T2dm): develop as a result of deterioration of β-cell function and subsequent reduction in insulin secretion capacity. Impaired fasting glucose is defined as fasting blood glucose of ≥100 and <126 mg/dL and impaired glucose tolerance is diagnosed if blood glucose is ≥140 and <200 mg/dL at the 2-h mark of the oral glucose tolerance test (OGTT) [1].
Non Alcoholic Hepatic Steatos: Is the most common cause of liver disease in children. Nonalcoholic Fatty Liver Disease (NAFLD) is considered part of a spectrum of the metabolic syndrome, as are other metabolic risk factors like high blood pressure, impaired glucose tolerance, type 2 diabetes (T02D), insulin resistance, dyslipidemia and visceral adiposity as early as during childhood [36]. In keeping with this, (Manco. et al. [37]). showed that at least 1 metabolic risk factor was present in the majority of children with biopsy-proven NAFLD. NAFLD is strongly associated with a number of metabolic risk factors, including insulin resistance, dyslipidaemia, cardiovascular disease and, most significantly, obesity [38]. Insulin Resistance (IR) represents the critical connector linking NAFLD and MetS in obese children and adolescents (Prokopowicz, et al. [39,40]). observed an impaired metabolic profile, characterized by greater waist circumference, IR, glucose dysregulation, and dyslipidemia in 45% of overweight adolescents with hepatic steatosis than children without. Moreover, 40.8% of children with NAFLD presented MetS.
Polycystic Ovary Syndrome (PCOS): Is a common disorder characterized by hyperandrogenism and disordered gonadotropin secretion, often associated with insulin resistance [41]. Twelve articles were finally included in the systematic review and meta-analysis by (Fu L, et al. [42]). About: The Association Between Polycystic Ovary Syndrome and Metabolic Syndrome in Adolescents. The results suggested that adolescents with PCOS have more than three times the odds of having MetS than controls (OR 3.32, 95% CI [2.14, 5.14]). Obese adolescents with PCOS also had a higher risk of MetS than those with obesity but without PCOS (OR 3.97, 95% CI [1.49, 10.53]). Compared to those without PCOS, systolic blood pressure was higher in adolescents with PCOS (weighted mean difference (WMD) 3.85, 95% CI [1.73, 5.97]), while diastolic blood pressure was higher only in girls with PCOS who had a normal weight (WMD 3.52, 95% CI [1.57, 5.48]). The levels of triglycerides were higher in obese adolescents with PCOS than in those with obesity but without PCOS (WMD 27.84, 95% CI [10.16, 45.51]). PCOS could increase the frequency of MetS by influencing blood pressure and lipid metabolism independent of obesity as early as the adolescent period.
Inflammatory Markers: Include interleukin-6, tumor necrosis factor-alpha, and C-reactive protein (CRP) [43]. C reactive protein is a sensitive marker of subclinical inflammation. C-reactive protein is associated with obesity, diabetes mellitus and increased cardiovascular risk [44]. In the study of Kitsios K, Papadopoulou M, et al High-sensitivity C-reactive protein (HsCRP) levels were significantly increased in obese and overweight subjects as compared to the control group, (0.61±08 vs. 0.05±0.18 mg/dL, p<0.001 and 0.33±0.25 vs. 0.05±0.18 mg/dL, p<0.001, respectively). HsCRP levels were similar between obese and overweight subjects (p=0.109). Obese and overweight children with NAFLD had significantly higher levels of hsCRP compared to their counterparts without NAFLD (0.78±1.4 vs. 0.34±0.31 mg/dL, p=0.016). The levels of hsCRP were comparable in the obese and overweight children/adolescents with and without MS and with or without prediabetes (0.95±1.66 vs. 0.35±0.27 mg/dL, p=0.096 and 0.43±0.34 vs. 0.53±1.0 mg/dL, p=0.589, respectively) [45] (Tables 5-9).
Table 5: Comparison of three diagnostic criteria for metabolic syndrome given by Cook et al, de Ferranti et al and Weiss et al [12].
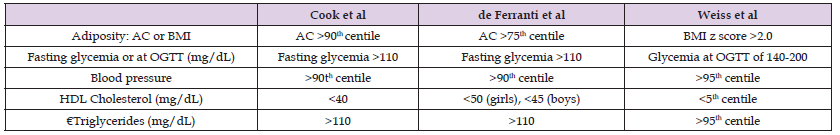
Table 7: The IDF definition of the at-risk group and the metabolic syndrome in children and adolescents (2007)
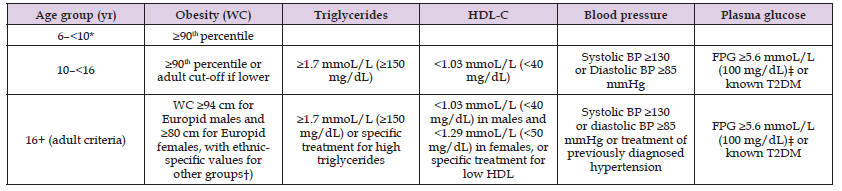
The diagnosis is based on the use of several different definitions of metabolic syndrome in children and adolescents. *The metabolic syndrome cannot be diagnosed, but further measurements should be made if there is a family history of the metabolic syndrome, type 2 diabetes, dyslipidemia, cardiovascular disease, hypertension, and/or obesity. †For those of South and South-East Asian, Japanese, and ethnic South and Central American origin, the cutoffs should be ≥90 cm for men, and ≥80 cm for women. The IDF Consensus Group recognize that there are ethnic, gender and age differences, but research is still needed on outcomes to establish risk. ‡For clinical purposes, but not for diagnosing the metabolic syndrome, if fasting plasma glucose is 5.6–6.9 mmoL/L (100–125 mg/dL) and it is not known to have diabetes, an oral glucose tolerance test should be performed. (6) Diagnosing the metabolic syndrome requires the presence of central obesity plus any two of the other four factors [46]. Dyslipidemia: an increased triglyceride to HDL ratio could be used as a marker for elevated low-density lipoprotein (LDL) in adolescents. A ratio of 3 or more, is indicative of more small-dense LDL particles and is associated with a higher risk for CVD given the atherogenic effect of this LDL [46]. According to the American Diabetes Association, diabetes is diagnosed when one of the following criteria is met: HbA1C ≥ 6.5% or essential glycemi ≥ 126mg/dL or glycemi 2 hours after meals ≥200mg/dL or random measurement of glycemia ≥ 200mg/dL [47].
Based on expert opinion, the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) recommends screening for nonalcoholic fatty liver disease (NAFLD) between 9 and 11 years of age with alanine aminotransferase (ALT) levels in obese children or in overweight children with additional risk factors. This differs from the American Association for the Study of Liver Diseases Practice Guidance, which does not recommend screening in children because of a paucity of data. In contrast, there are no recent screening guidelines from the American Academy of Pediatrics. In short, consistent guidelines and additional data are needed to improve NAFLD screening and diagnosis among children [48]. The diagnosis of NAFLD should be actively considered in all overweight or obese children >10 years old, particularly in the context of hypertension, evidence of hepatomegaly, acanthosis nigricans, insulin resistance and Type II diabetes mellitus [49]. Transaminases of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are of clinical value. Both of these markers, but especially ALT, are commonly used in clinical medicine as indicators of hepatic damage. They are useful screening tests for hepatic steatosis [50] children over the age of 6 should be screened at least once a year with a BMI. An obesity screening may use a measurement called a BMI (body mass index) and other tests to find out if the child is overweight or has obesity. The results will be in the form of a percentile. A percentile is a type of comparison between an individual and a group.
For example, if the child has a BMI in the 50th percentile, it means 50 percent of children of the same age and sex have a lower BMI, and 50 percent will have a higher BMI. The child's BMI will show one of the following results: Less than the 5th percentile: Underweight,5th-84th percentile: Normal Weight,85th-94th percentile: Overweight, 95th percentile and higher: Obese [51]. Lipid profile should be repeated between age 12 and 16 years in overweight adolescents per the NHLBI recommendations [1]. Strategies for screening for prediabetes and type 2 diabetes generally involve targeted screening of children who are overweight or obese for the presence of one or more risk factors (e.g., type 2 diabetes in a first- or second-degree relative, member of a high-risk racial/ethnic group. The USPSTF recommends that clinicians screen for obesity in children and adolescents age 6 years or older and offer or refer them to comprehensive, intensive behavioral interventions to promote improvements in weight status. The American Diabetes Association (ADA) recommends risk-based screening for type 2 diabetes in children after onset of puberty or age 10 years who are overweight (BMI ≥85th percentile) or obese (BMI ≥95th percentile) and have one or more additional risk factors for diabetes. Such additional risk factors include maternal history of diabetes or gestational diabetes mellitus during the child’s gestation; family history of type 2 diabetes in first- or second-degree relative; being a member of a high-risk racial/ethnic group, including Native American, African American, Latino, Asian American, and Pacific Islander; or signs of insulin resistance or conditions associated with insulin resistance, including acanthosis nigricans, hypertension, dyslipidemia, polycystic ovary syndrome, or small-for-gestational-age birth weight.
In terms of screening frequency, the ADA recommends screening to be repeated every 3 years if tests are normal or more frequently if BMI increases. The ADA recommends testing with fasting plasma glucose, 2-h plasma glucose (PG) after 75-g OGTT, or an A1c. Further, the ADA recommends that children and adolescents who are overweight or obese for whom the diagnosis of type 2 diabetes is being considered have a panel of pancreatic autoantibodies tested to exclude the possibility of autoimmune type 1 diabetes [52]. The oral glucose tolerance test (OGTT) is currently the gold standard for the diagnosis of diabetes. The recommended preparation for and administration of the OGTT are important to ensure that test results are not affected. Interpretation is based on venous plasma glucose results before and 2 hours after a 75 g oral glucose load [53].
In general, the therapeutic interventions are divided into lifestyle modification, pharmaceutical therapy, and bariatric surgery [6]. The primary goal in prevention of pediatric obesity, a big indicator in metabolic syndrome is promoting life style modifications such as healthy diet and increased physical activity. Life style modifications include adopting healthy eating habits by increasing intake of fruits and vegetables, more fiber and less dietary fat in addition to avoiding carbonated drinks, refined carbohydrates, high fructose corn syrup, high sodium, and processed food [1]. Low sodium intake has a good benefit for blood pressure regulation [6]. Fruit juice intake should be limited to 4–6 ounces per day for children 6 month to 6 years of age and 8–12 ounces daily for older children according the American Academy of Pediatrics (AAP). Daily fruit juice intake is found to be associated with increased risk for developing overweight status and obesity especially in early preschool years [1]. Overconsumption of fast foods in combination with inactive physical activity is strongly associated with the high prevalence of overweight, obesity, dyslipidemia, hypertension, type 2 diabetes, and cardiovascular disease in children and adolescents over the past 30 years. Clearly, eating healthy diet has a significant impact on all the components of the metabolic syndrome [6].
Careful monitoring of infant growth to detect the early development of obesity would seem important. Given the evidence of widespread benefits which arise from breast-feeding, promotion of exclusive breast-feeding for the first four to 6 months of life would seem worthwhile. After weaning, infants should be exposed to a diverse diet and from the age of 1 year, infants should feed with the rest of the family. A combination of enhanced physical activity and improved nutrition seem most likely to be beneficial in preventing obesity. A number of international guidelines have advocated a range of measures to reduce the risks of developing obesity. These include promotion of moderate to intensive physical activity by encouraging a minimum of 20 min but preferably more activity (about one to one and a half hours and equivalent to at least 10–12,000 steps daily. These targets should be achieved at least 5 days weekly as a core aim. Promotion of active methods such as cycling for travel to school are advised [54]. At the same time, interventions that reduce screen (television, computer, or smart phone) time may be effective ways of targeting sedentary behavior; screen time should be reduced to a maximum of 2 h daily. The US Preventative Services Task Force recommends behavioral interventions to reduce sedentary screen time among children 13 years and younger [54].
Adopting healthy sleep habits is very important. Disordered sleep length and quality affect appetite and decrease insulin sensitivity. The National Sleep Foundation recommends 8–11 h of sleep for school age children and adolescents [55]. So preventative strategies are likely to be most successful when targeting the young and will require a combination of approaches which will need inter-disciplinary collaborations across health and local government to target families, schools, and local environments to facilitate behavior changes which influence young people's eating behaviors and habitual levels of physical activity [54] (Table 10).
Medications to treat Diabetes Mellitus Type 2(T2DM), hypertension and dyslipidemia should be initiated as appropriate. Pharmacological therapy for obesity are becoming increasingly common, particularly among those who do not respond to behavioral therapy alone. The US Food and Drug Administration (FDA) has approved pharmacotherapy for children or adolescents with obesity, which is to be considered after a formal program of intensive lifestyle modification has failed to help with weight reduction or for management of comorbidities. High intensity life style modification programs should be continued along with pharmacotherapy. In general, FDA approved medications used in adults are also approved for adolescents ≥16 years of age with a BMI of ≥30 or ≥27 kg/m2 associated with at least one obesity-related comorbidity. Adolescents undergoing treatment with anti-obesity medications should be monitored throughout the therapy period for side effects but also for weight loss [1]. In cases with documented hyperinsulinemia and insulin resistance, even if the glucose intolerance has not reached diabetic level, metformin [12]. an effective anti-hyperglycemic agent [56] may be tried to improve insulin sensitivity, reduce obesity, acanthosis nigricans and symptoms of polycystic ovarian syndrome if present [12]. Metformin is not approved for use in those aged, 18 years in Canada. In the United States, metformin is the only approved oral medication for use in children aged. 10 years with type 2 diabetes [57]. Metformin appears to be safe in children and adolescents with little risk of hypoglycemia and/or lactic acidosis [56] Adeyemo et al found that, compared to placebo-treatment, metformin reduced body mass index (BMI) by an average of 1.09 kg/m2, and body weight by 3.38 kg, in 6–12y overweight and insulin-resistant children over a 6-month period [58].
Glimepiride, glibenclamide and gliclazide are other oral hypoglycaemic agents sometimes used in adolescents with T2DM.However, prevalence of type 1 diabetes mellitus (T1DM) is many times higher in children as compared to T2DM, for which insulin injection is the main treatment option utilized to achieve glycemic control [12]. Only orlistat is indicated for the treatment of overweight adolescents. It is approved by the FDA for the treatment of obesity in adolescents aged 12 years and older [59]. Gastrointestinal side effects are common and may limit use [60]. In the Summary of 2014 National Institute for Health and Care Excellence guidance for prescribing of orlistat to children and young people: Drug treatment is not generally recommended for children younger than 12 years. In children younger than 12 years, drug treatment may be used only in exceptional circumstances, if severe comorbidities are present. Prescribing should be started and monitored only in specialist paediatric settings. In children aged 12 years and older, treatment with orlistat is recommended only if physical comorbidities (such as orthopaedic problems or sleep apnoea) or severe psychological comorbidities are present. Treatment should be started in a specialist paediatric setting, by multidisciplinary teams with experience of prescribing in this age group. Orlistat shouldn’t be given to children for obesity unless prescribed by a multidisciplinary team with expertise in drug monitoring, psychological support, behavioural interventions, interventions to increase physical activity and interventions to improve diet. Drug treatment may be continued in primary care, for example, with a shared care protocol if local circumstances and/or licensing allow [61]. Conjugated linoleic acid (CLA) is thought to be a weight reduction promoting agent, however based on meta-analysis by Onakpoya and colleagues, there is no evidence to support that CLA intake has any clinically relevant effects on body composition over long term. It was noticed that CLA has minimal weight loss effects [1].
Pre-Requisites for Bariatric Surgery
Metabolic and bariatric surgery are existing but underuse treatment options for pediatric patients with severe obesity [61]. Bariatric surgery are only rarely preferred especially for pathological obesity and the prerequisites are child with BMI>40 or >35 with significant comorbidities, child has attained Tanner 4/5 pubertal development and bone age of >13 years (girls) and 15 years (boys), failure of all other modalities of treatment, strong family support and strong will to adhere to treatment both before and after surgery [12]. The comorbidities substantiating the indication are non-alcoholic liver steatosis, T2DM, obstructive sleep apnoea syndrome, cardiovascular risk factors, orthopaedic disease, physical impairment, gastro-oesophageal reflux disease, idiopathic intracranial hypertension, and low quality of life [62]. Exclusion criteria for bariatric surgery in adolescents include: Severe psychiatric disorders (unstable psychosis, borderline personality, severe depression and personality disorders, active suicidality) and diagnosed eating disorders; alcohol and/or drug abuse; pregnancy (present or planned within 18 months after surgery); inability of the patient to participate in a long-term interdisciplinary follow-up at the obesity center [63]. Vitamin levels should be monitored before and after metabolic and bariatric surgery (MBS) with all attempts to maximize adherence with vitamin supplements long term. Multidisciplinary teams should stabilize and treat preexisting eating disorders, assure stable social support, assess and assist with nutrition and activity knowledge, and consider the addition of medications when appropriate. Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program (MBSAQIP) guidelines should be followed when building an adolescent metabolic and bariatric surgery (MBS) program. It is the responsibility of the adolescent MBS program to have a transition plan in place for adolescents to transition to an adult MBS program for lifelong care [64-68].
Children with metabolic syndrome have an increased risk of metabolic syndrome as adults, and possibly an increased risk of type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD). Al-Hamad
Dietary therapy and increasing physical activity are cornerstones of management. Panda P K.
Metabolic and bariatric surgery (MBS) should be a considered standard of care for adolescents with severe obesity. Bolling CF.
This work was carried out in collaboration among all authors. FP designed the study and wrote the manuscript. ER, ZM, are the treating clinicians that performed clinical evaluation and follow-up. EP revised the literature and rewrote the manuscript. All authors read and approved the final manuscript.


