Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Boyun Shi1,2#, Wei Wang1#, Yanpeng Wu1,2#, Ziyu Yu1#, Mengting Ye1, Yingying Zhang1, Junting Huang3*, Kailian Hou1,4*, Xinke Zhou1* and Leyuan Liu1*
Received: June 01, 2023; Published: June 09, 2023
*Corresponding author: Leyuan Liu, The Key Laboratory of Biological Targeting Diagnosis, Therapy and Rehabilitation of Guangdong Higher Education Institutes. The Fifth Affiliated Hospital, Guangzhou Medical University, Guangzhou, Guangdong, 510700, P. R. China
Xinke Zhou, The Key Laboratory of Biological Targeting Diagnosis, Therapy and Rehabilitation of Guangdong Higher Education Institutes, The Fifth Affiliated Hospital, Guangzhou Medical University, Guangzhou, Guangdong, 510700, P. R. China
Kailian Hou, The Key Laboratory of Biological Targeting Diagnosis, Therapy and Rehabilitation of Guangdong Higher Education Institutes. The Fifth Affiliated Hospital, Guangzhou Medical University, Guangzhou, Guangdong, 510700, P. R. China and Department of Oncology, The Fifth Affiliated Hospital, Guangzhou Medical University,
Guangzhou, Guangdong, 510700, P. R. China
Junting Huang, Department of Pediatric Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-Sen University Cancer Center, Guangzhou, Guangdong, 510060, P. R. China
DOI: 10.26717/BJSTR.2023.51.008032
Background: Hepatoblastoma (HB) is an embryonic tumor and the most common malignant tumor in the liver of children. Currently, tumor recurrence and metastasis are still the primary causes of death and the key deterrents for the long-term survival of HB patients. Microtubule-associated protein MAP1S supports intracellular trafficking along microtubules and suppresses hepatocellular carcinoma by promoting autophagy.
Methods: In order to explore roles of MAP1S in the development of HB, we measured the levels of MAP1S protein in liver tissues collected from HB patients and analyzed the impact of MAP1S in cultured cancer cells.
Results: We found that the levels of MAP1S protein in HB tissues were significantly lower than those in their adjacent non-tumor tissues, and survival rates of patients with high levels of MAP1S were significantly higher than those with low levels of MAP1S. When levels of MAP1S were suppressed, levels of β-catenin, mesenchymal markers Vimentin and N-cadherin were increased but the levels of epithelial marker E-cadherin were decreased at both mRNA and protein levels, suggesting the processes of epithelial to mesenchymal transition (EMT) and rates of proliferation and invasion of HB cells were significantly enhanced. Especially, MAP1S regulated levels of mesenchymal markers through autophagy.
Conclusion: MAP1S prevents metastasis and improves survival of HB patients by suppressing EMT.
Keywords: Autophagy; EMT; Hepatoblastoma; MAP1S; Metastasis; Prognosis
Hepatoblastoma (HB) is the most common malignant liver tumors in children although the etiology of HB is currently unclear [1,2]. It has been shown that HB usually occurs in children with preexisting genetic disorders such as Beckwith-Wiedemann syndrome, familial adenomatous polyposis and trisomy 18 [3,4]. The clinical symptoms of children with HB are mostly abdominal masses and some non- specific symptoms such as anorexia and weight loss; and about 90% of patients in laboratory examinations have elevated serum alpha-fetoprotein (AFP) levels [5]. Histologically, HB can be classified as well-differentiated fetal, embryonal, macrotrabecular and small cell undifferentiated subtypes [6]. The incidence of HB is increasing at a rate higher than any other pediatric malignancy worldwide, and the best treatment for the survival of patients with progressive and metastatic HB is surgical resection [7]. However, children with malignant HB being too late to do surgical resection or losing the chance to receive liver transplantation only have a 5-year survival rate around 20% [8]. Therefore, to understand the biological mechanism leading the HB development and search for new prognostic biomarkers to help assess disease risks and formulate treatment plan and improve the overall survival rate of children have become the focus of current research.
The widely distributed MAP1S, originally named C19ORF5, is a member of microtubule- associated protein family 1 which also includes the neuron-distributed MAP1A and MAP1B [9,10]. MAP1S can connect mitochondria and microtubules for transport, and acts as a bridge between the autophagy mechanism and microtubules [10,11]. Deleting MAP1S gene in mice leads to a blockade of autophagy flux, which in turn increases oxidative stress and genomic instability resulting in the HCC initiation and development [11,12]. Inversely, maintaining the acetylation and stability of MAP1S with spermidine in mice results in the suppression of HCC as well as liver fibrosis in addition to the prolongation of lifespan [13,14]. Based on clinical samples, MAP1S was characterized to be a suppressor and a potential prognosis marker of prostate adenocarcinomas, clear cell renal cell carcinomas and pancreatic ductal adenocarcinomas [15-17]. In addition, MAP1S was reported to activate autophagy flux to suppress liver fibrosis [18] and renal fibrosis [19] and degrade the aggregates of mutant huntingtin proteins associated with Huntington’s disease [20]. It is seemly that MAP1S is a universal tumor suppressor and may play a similar role in HB development. Here, we determined levels of MAP1S protein in HB and their adjacent tissues from HB patients and found that MAP1S deficiency was similarly associated with metastasis and poor prognosis. Suppressing MAP1S led to a promotion of epithelial-mesenchymal transition (EMT) by regulating the mRNA and protein levels of EMT-associated proteins and enhancements of proliferation, migration and invasion of HB cell HepG2. Therefore, MAP1S prevents metastasis and enhances survivals of HB patients by suppressing EMT.
Collection of Liver Tissues from HB Patients
An ethical approval for human subjects was obtained from the Research and Ethics Committees of The Fifth Affiliated Hospital of Guangzhou Medical University and Sun Yat-sen University Cancer Center. Sixty-two HB tissues and 15 normal tissues were collected from HB patients with age range from 1 to 110 months and the median age of 25.5 months undergone curative liver resection in the hospitals between April 2015 and December 2017 with consent in writing to donate tissue specimens for the intended research from patients themselves or members of their families. The diagnoses were confirmed by histological reviews and patients had been followed after surgery until August 2019 for a complete set of clinical data assessed by two independent clinical pathologists in a double-blinded manner as shown in Table 1. Risk stratification and chemotherapy regimens for children with HB were based on a CCCG-HB-2016 protocol in Chinese [21]. Very low-risk patients did not undergo chemotherapy before and after surgery, low-risk ones were treated with C5V regimen (cisplatin + fluorouracil + vincristine) chemotherapy for a total of 4 cycles after surgery, medium risk ones were treated with C5VD regimen (cisplatin + fluorouracil + vincristine + doxorubicin) chemotherapy for a total of 6-8 cycles preoperative and postoperative, and high-risk patients underwent 8 cycles of chemotherapy with C5VD regimen and IIV regimen (ifosfamide + irinotecan + vincristine) before and after surgery. The collected tissues were fixed in 4% paraformaldehyde at 4°C overnight, dehydrated, processed, paraffin -embedded, and then sectioned in 5-μm thickness for hematoxylin and eosin staining and immuno-histochemical analyses.
Note: *including fetal, embryonic and small cell undifferentiated type.
Immunohistochemical Staining of Liver Tissue Sections from HB Patients
The tissue sections were prepared for immunochemical staining following the standard procedures as we previously reported [13,15,18,22]. Tissue sections were then incubated with antibody against human MAP1S (Cat# 15695-1-AP, Protein tech Group, Inc, Rosemont, IL, USA, 1:200 dilution), β- catenin (Cat#: 8480, Cell Signaling Technology (CST), Danvers, MA, USA, 1:100 dilution), E-cadherin (Cat#: 3195, Cell Signaling Technology (CST), Danvers, MA, USA, 1:100 dilution), N-cadherin (Cat#: 13116, Cell Signaling Technology (CST), Danvers, MA, USA, 1:100 dilution) or Vimentin (Cat#: sc- 47778, Protein tech Group, Inc, Rosemont, IL, USA, 1-100 dilution) at 4°C overnight. After washing, the sections were reacted with a second antibody of 1:5000 anti-rabbit-HRP (Cat# BS13278, Bioworld Technology, Inc, Bloomington, MN, USA). Images were captured using a Leica DM6 B upright microscope (magnification, 200X). Image-Pro Plus version 6.0 software. Media Cybernetics, Inc., Rockville, MD, USA) was used to assess the staining area and intensity and generate an integrated optical density (IOD) value for each image. Five randomly selected images from each tissue section were captured and the mean of IOD values of 5 images was designated as the representative IOD for such tissue section. IOD values larger than or equal to 0.59 were considered as high and smaller than 0.59 were considered as low. The difference in IOD of proteins between normal tissues and HB tissues was tested with Student’s t-test, and the differences in MAP1S intensities classified as high or low between different clinicopathological variables were tested with chi-square test. The overall survival was measured from the start of treatment until the end of the observation period and analyzed by the Kaplan- Meier method. Cox proportional-hazard analysis with univariate or multivariate method was used to explore the impact of MAP1S on overall survivals and event-free survivals of HB patients with the GraphPad Prism 9.0 software.
Establishment of a Stable MAP1S-/- Cell Line and Assays of Cell Proliferation, Migration and Invasion
Human HB cell HepG2 authenticated by STR profiling and tested for mycoplasma contamination was purchased from Shenzhen Jiabaishun Technology Co., LTD (Shenzhen, Guangdong, China) to establish a stable cell line with CRISPR/Cas9 technique in a similar way as described [20]. Because of the potential dramatic drifting in genetic background in the selection of single cell to establish a cell line, we have maintained a mixture of transfected cells after selection. The sequences of the DNA oligos for the gRNAs were MAP1Sg3- F: 5′-CACCGGGGCTCCTCACCTACGTCC-3′ and MAP1S-g3-R: 5′-AAACGGACGTAGGTGAGGAGCCCC-3′. Cells were cultured in highglucose Dulbecco’s Modified Eagle’s Medium (DMEM) (HyClone, USA) supplemented with 10% FBS (Gibco, Gaithersburg, MD, USA) and 1% penicillin/streptomycin (Solarbio, China) under 5% CO2 at 37℃. Cell proliferation assays by measuring the OD values at 450 nm and number of EdU-labelled cells and migration and invasion assays by transwell experiments and cell scratch experiments were conducted as previously reported [23,24]. For CCK-8 viability assay, cells were seeded into a 96-well plate at a density of 4X103 cells per well for 24, 48, or 72 hrs. Cell counting kit-8 (CCK8) reagent (Dojindo, Japan) was added at a dilution of 1:10 to each well and incubated for 2 hrs. The absorbance was then measured at a wavelength of 450 nm using an Ultra-micro spectrophotometer (BioTek, USA). For Edu proliferation assay, cells were seeded at a density of 5 × 104 cells/mL into 24- well culture plates. The EdU staining was conducted using a Cell- Light EdU DNA Cell Proliferation Kit (RiboBio, Guangzhou, China) according to the manufacturer’s protocol. Cells were incubated with EdU (1:1000) for 8hrs before being harvested by rinsing in PBS, fixed with paraformaldehyde and permeabilized with 0.3% Triton X-100 in PBS for 10 min, and washed.
Cells were incubated with Apollo staining reaction solution for 30 mins in the dark. Next, cells were nuclear stained with DAPI for 5 mins and images were captured using an Olympus laser scanning microscope system. The experiment was independently repeated three times. Trans well assays for vertical migration and invasion were performed using trans well chambers with 8-μm pores (Corning Incorporated, Corning, NY, USA). A total of 5 × 104 cells were resuspended in serum-free medium and seeded in the upper chamber, while the lower chamber was filled with complete medium. After a 48- hr incubation, the cells in the upper chamber were carefully removed with a cotton swab, and the cells that had migrated through the membrane to the lower surface were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. For invasion assay, Matrigel (BD Biosciences) was pre-coated on the membrane of the upper chamber and cells were seeded as migration assay and allowed to invade for 48 hrs. The cell count was done under the microscope (100X). Data were presented as the means of migrated cells in five randomly chosen fields. Wound healing assay for horizontal migration was initiated with an artificial homogenous wound created onto the monolayer with a sterile 10-μl tip. After scratching, the culture dishes were washed with serum-free medium. Images of cells migrating into the wound were captured at time 0, 24, 48 and 72hrs by an inverted microscope (100X), and the area of scratch was measured by the ImageJ software (National Institutes of Health, Bethesda, MD, USA). The differences in cell proliferation, migration and invasion between wild-type and MAP1S knockout cells at each time points were tested using Student’s t test.
Immunoblot Analyses
HepG2 cells or Huh-6 cells purchased from Genetic Testing Biotechnology Corporation (Suzhou, Jiangsu, China) with MAP1S transiently suppressed by transfection performed as previously described before [13,18,20] were untreated or treated with 2μM Bafilomycin A1 (Cat#: HY-100558, Med Chem Express (MCE), Monmouth Junction, NJ, USA). Cell lysates were prepared to conduct immunoblot analyses of the levels of EMT-associated proteins and autophagy flux as previously described [23]. Proteins were separated through electrophoresis, probed with primary antibodies against MAP1S (Cat# 15695-1-AP, Protein tech Group, Inc, Rosemont, IL, USA, 1:4000 dilution), β-catenin (Cat#: 8480, Cell Signaling Technology (CST), Danvers, MA, USA, 1:1000 dilution), E-cadherin (Cat#: 3195, Cell Signaling Technology (CST), Danvers, MA, USA, 1:1000 dilution), N-cadherin (Cat#: 13116, Cell Signaling Technology (CST), Danvers, MA, USA, 1:1000 dilution), Vimentin (Cat#: sc-47778, Protein tech Group, Inc, Rosemont, IL, USA, 1-50000 dilution), LC3-II (Cat#: 12741, Cell Signaling Technology (CST), Danvers, MA, USA, 1:1000 dilution), GAPDH (Cat#: sc-47724, Santa Cruz Biotechnology, Dallas, Texas, USA, 1-1000 dilution) and β-actin (Cat#: AP0060, Bio world Technology, Inc, Bloomington, MN, USA, 1-10000 dilution) and their respective horseradish peroxidase (HRP)- conjugated secondary antibody Goat anti-Rabbit IgG(H&L)-HRP (Cat#: BS13278, Bio world Technology, Inc, Bloomington, MN, USA, 1-10000 dilution) or Goat anti-Mouse IgG(H&L)-HRP (Cat#: A0216, Beyotime Biotechnology, China, 1-1000 dilution), incubated with Immobilon Western Chemiluminescent HRP Substrate (Cat#:WBKLS0500, Millipore, Decatur, IL, USA), and visualized with Molecular Imager (Model No: Universal Hood II Serial No: 721BR 13945, BIO-RAD, Hercules, CA, USA). The intensities of protein bands were quantified using ImageJ software.
RT-qPCR Analyses of mRNA Levels of EMT-Associated Proteins
The impact of MAP1S suppression on the mRNA levels of EMTassociated proteins was assayed by RT-qPCR technique with specific pairs of primers for MAP1S: MAP1S-F (5’- gTgTTCATCCCCCATCCCTC-3’) and MAP1S-R (5’-ACTCCAggAATTCgCACAgg-3’); β-catenin: β-catenin-F (5’-CTgAggAgCAgCTTCAgTCC-3’) and β-catenin-R (5’-ATTgCACgTgTggCAAgTTC-3’);E-cadherin: E-cadherin-F (5’-TCTCCCTgCAgTgAATTTTgA-3’) and E-cadherin-R (5’-TTTgAATCgggTgTCgAggg-3’); N-cadherin: N-cadherin-F (5’-TgCCCggTTTCATTTAgggg-3’) and N- cadherin-R (5’-ACTAACCCgTCgTTgCTgTT-3’); Vimentin: Vimentin-F (5’- AgTCCgCACATTCgAgCAA-3’) and Vimentin-R (5’-CgggTgTTCTTgAACTCggT-3’); and GAPDH: GAPDH-F (5’-AggCTAgCTggCCCgATTTC-3’) and GAPDH-R (5’-TggCAACAATATCCACTTTACCAgA-3’). Total RNA was isolated using TRIzol reagent (Invitrogen, USA), cDNA was generated by Prime Script RT Master Mix (TaKaRa, Tokyo, Japan), and quantitative realtime PCR was performed using SYBR-green PCR Master Mix (TaKaRa). PCR assays were performed three times, and the expression of the genes was calculated using the comparative Ct method.
Levels of MAP1S in HB Tissues are Significantly Suppressed
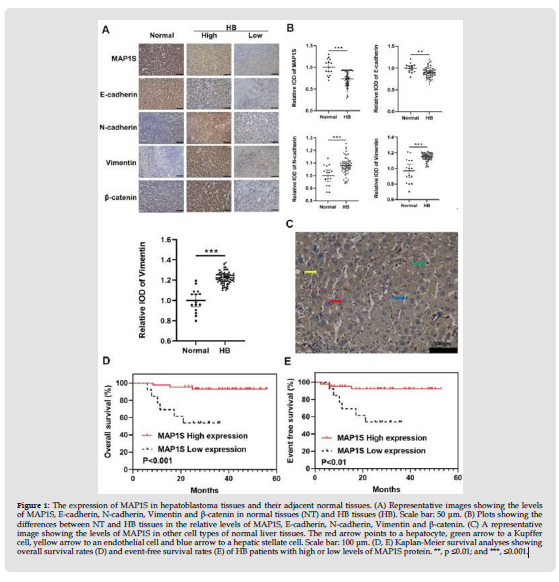
We collected 62 HB tissues and 15 normal liver tissues from HB patients with age range from 1 to 110 months and the median age of 25.5 months who were enrolled in our hospitals and conducted immunohistochemical staining to detect the levels of MAP1S protein. It was found that MAP1S was mainly expressed in the cytoplasm; levels of MAP1S in normal tissues were uniformly high while those in HB tissues were variable in a range from positive as the similar levels to those in normal tissues to negative; and levels of MAP1S in normal tissues were significantly higher than those in HB tissues (Figures 1A & 1B). It was also found that other cell types in normal liver tissues expressed relatively similar levels of MAP1S (Figure 1C). Therefore, it is suggested that MAP1S is potentially a suppressor of HB.
Reduced Levels of MAP1S are Closely Associated with Metastasis and Poor Prognosis of HB Patients
In order to explore the clinicopathological significance and function of MAP1S in HB, we analyzed the correlation between the levels of MAP1S and clinicopathological features and survival rates of HB patients. Patients with low levels of serum AFP, low CHIC risk or without metastasis exhibited significantly higher levels of MAP1S than those with high levels of serum AFP, high CHIC risk or metastasis (Table 1). Univariate Cox proportional hazard analyses indicated that low levels of MAP1S, high CHIC risk and metastasis significantly impaired the overall survivals and event-free survivals of HB patients (Table 2). Multivariate Cox proportional hazard analyses revealed that only low levels of MAP1S significantly impaired the overall survivals of HB patients (Table 3). Kaplan-Meier survival analyses revealed that patients with high levels of MAP1S in HB tissues had significantly higher rates of overall and event-free survival than those with low levels of MAP1S (Figures 1D & 1E). Therefore, MAP1S may suppress HB metastasis and enhance patients’ survivals.
Figure 1 The expression of MAP1S in hepatoblastoma tissues and their adjacent normal tissues. (A) Representative images showing the levels of MAP1S, E-cadherin, N-cadherin, Vimentin and β-catenin in normal tissues (NT) and HB tissues (HB). Scale bar: 50 μm. (B) Plots showing the differences between NT and HB tissues in the relative levels of MAP1S, E-cadherin, N-cadherin, Vimentin and β-catenin. (C) A representative image showing the levels of MAP1S in other cell types of normal liver tissues. The red arrow points to a hepatocyte, green arrow to a Kupffer cell, yellow arrow to an endothelial cell and blue arrow to a hepatic stellate cell. Scale bar: 100 μm. (D, E) Kaplan-Meier survival analyses showing overall survival rates (D) and event-free survival rates (E) of HB patients with high or low levels of MAP1S protein. **, p ≤0.01; and ***, ≤0.001.
Note: CI: Confidential Interval; EFS: Event-Free Survival; HR: Hazard Ratio; OS: Overall Survival
Note: CI: Confidential Interval; EFS: Event-Free Survival; HR: Hazard Ratio; OS: Overall Survival
MAP1S Suppresses the Proliferation, Migration and Invasion of HB Cells
Clinical results suggesting that MAP1S may suppress HB metastasis and further patient survivals triggered us to test the impact of MAP1S on proliferation, migration and invasion of representative HB cell HepG2. We created a stable HepG2 cell line with whose levels of MAP1S were significantly suppressed (Figures 2A & 2B). Cells with reduced levels of MAP1S exhibited significantly higher rates of proliferation as indicated by OD values at 450 nm (Figure 2C) and the number of EdU-labelled cells (Figures 2D & 2E) and faster rates of migration and invasion as indicated by transwell experiments (Figures 2F-2H) and scratch experiments (Figures 2I & 2J). Therefore, MAP1S indeed suppresses the proferliation, migration and invasion of HepG2 cells.
MAP1S Regulates Levels of EMT-Associated Proteins
The close association of EMT with cancer metastasis [25] triggered us to investigate the impact of MAP1S on cancer cell EMT. Examining the expression levels of EMT-associated proteins in HB tissues indicated that the levels of epithelial cell marker E-cadherin were significantly reduced and mesenchymal cell markers N-cadherin and Vimentin were significantly increased in HB tissues (Figures 1A & 1B). Since the activation of β-catenin-controlled signal pathways is the key initial step of EMT [26] and levels of β- catenin in HB tissues were significantly increased (Figures 1A & 1B), we tested the direct impact of MAP1S on β-catenin in HepG2 cells and found that MAP1S suppression (Figures 3A & 3B) led to significant increase in the levels of β-catenin protein (Figures 3A & 3C), suggesting an activation of EMT. The reduction in levels of E-cadherin (Figures 3A & 3D) and the increases in levels of N-cadherin and Vimentin (Figures 3E-3H) after MAP1S suppression further confirmed an activation of EMT. The same results were repeated in Huh-6 cells (Figures 3I-3K). The positive association between levels of MAP1S and levels of E-cadherin and negative association between levels of MAP1S and levels β-catenin, N-cadherin and Vimentin as well as the negative association between levels of E-cadherin and levels of β-catenin, N-cadherin and Vimentin were also observed in HB tissues (Figures 1A & 1B, Table 4). Therefore, MAP1S may suppress metastasis by promoting EMT.
MAP1S Regulates EMT at Both mRNA and Protein Levels
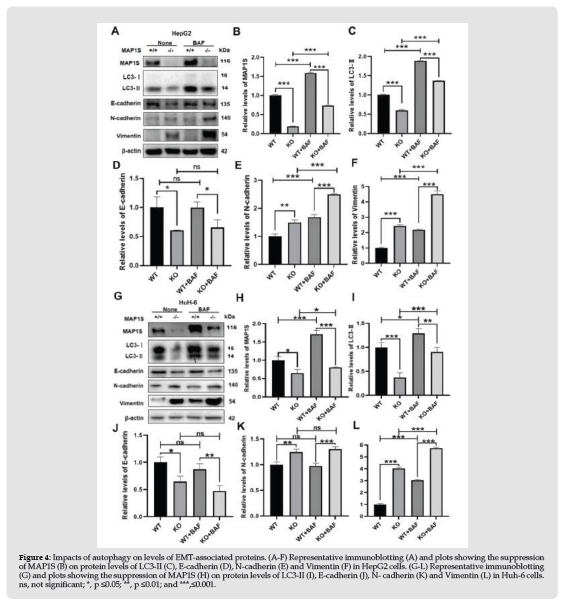
To further explore the mechanism by which MAP1S regulates EMT, we tested the impact of MAP1S on the mRNA levels of EMT-related marker genes. We found that cells expressing less MAP1S (Figure 3Q) expressed reduced levels of E-cadherin (Figure 3R) and increased levels of N-cadherin, β-catenin and Vimentin mRNAs (Figures 3S-3U). Although it is still unknown the exact mechanism how a microtubuleassociated protein MAP1S regulates the mRNA levels of these genes, it is sure that MAP1S regulates EMT at mRNA levels. As an autophagy regulator [10], we further tested whether MAP1S regulates through autophagy. As predicted, suppressing expression of MAP1S (Figures 4A & 4B) led to an impairment of autophagy flux in HepG2 cells (Figures 4A & 4C). The levels of E-cadherin protein were not changed upon autophagy blockade with lysosomal inhibitor Bafilomycin A1 (Figures 4A & 4D). The levels of mensenchymal marker N-cadherin and Vimentin were further increased in both wild type and MAP1S knockout cells after autophagy flux was blocked (Figures 4A,4E & 4F). The same results were repeated in Huh-6 cells (Figures 4G-4L). Therefore, MAP1S suppresses EMT at both mRNA and protein levels.
Figure 2 Impacts of MAP1S expression on the proliferation, invasion and migration of HB cell HepG2. (A, B) Representative immunoblotting (A) and plots (B) showing the levels of MAP1S in wild-type (+/+) and MAP1S knockout (-/-) HepG2 cell lines. (C) Plots showing the OD values of the CCK8 experiment of MAP1S+/+ and MAP1S-/- HepG2 cells. (D, E) Representative images (D) and plots (E) showing the number of EdU-labeling MAP1S+/+ and MAP1S-/- HepG2 cells. (F-H) Representative images (F) and plots showing number of migratory (G) and invasive (H) MAP1S+/+ and MAP1S-/- HepG2 cells in transwell experiments. (I, J) Representative images (I) and plots (J) showing the migration distance of MAP1S+/+ and MAP1S-/- HepG2 cells in scratch experiments. All plots here and after show the values of mean and standard deviation. *, p ≤0.05; and **, ≤0.01.
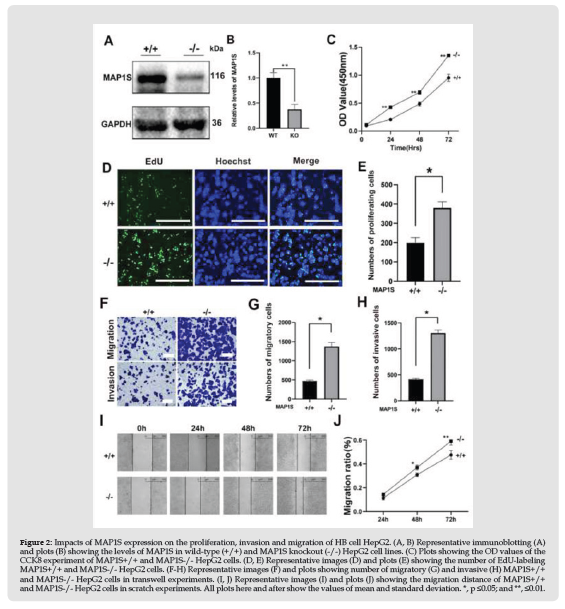
Figure 3 Impacts of MAP1S expression on levels of proteins related to EMT. (A-H) Representative immunoblotting (A) and plots showing the impacts of MAPP1S suppression (B) on epithelial cell marker proteins E-cadherin (C) and β-catenin (D) in HepG2 cells. (E-H) Representative immunoblotting (E) and plots showing the impacts of MAP1S suppression (F) on mesenchymal cell marker proteins N- cadherin (G) and Vimentin (H) in HepG2 cells. (I-L) Representative immunoblotting (I) and plots showing the impacts of MAP1S suppression (J) on proteins E-cadherin (K) and β-catenin (L) in Huh-6 cells. (M-P) Representative immunoblotting (M) and plots showing the impacts of MAPP1S suppression (N) on proteins N-cadherin (O) and Vimentin (P) in Hub-6 cells. (Q-U) Plots showing the suppression of MAP1S (Q) on the mRNA levels of E-cadherin (R), N-cadherin (S), β-catenin (T) and Vimentin (U). ns, not significant; *, p ≤0.05; **, p ≤0.01; and ***, ≤0.001.
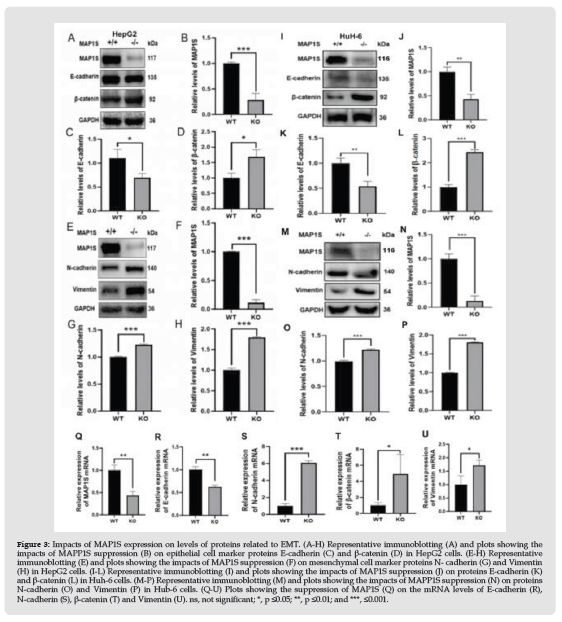
Figure 4 Impacts of autophagy on levels of EMT-associated proteins. (A-F) Representative immunoblotting (A) and plots showing the suppression of MAP1S (B) on protein levels of LC3-II (C), E-cadherin (D), N-cadherin (E) and Vimentin (F) in HepG2 cells. (G-L) Representative immunoblotting (G) and plots showing the suppression of MAP1S (H) on protein levels of LC3-II (I), E-cadherin (J), N- cadherin (K) and Vimentin (L) in Huh-6 cells. ns, not significant; *, p ≤0.05; **, p ≤0.01; and ***,≤0.001.
Most HB cases are sporadic although patients with certain genetic syndromes have higher risk to develop HB, unlike other types of cancers onset at adulthood which are frequently caused by patients’ lifestyles developed by themselves, HB is more likely caused by inherent nongenetic risk factors such as birth weight, parental age and congenital anomalies [4]. With the advance of medical technologies in adjuvant chemotherapy and hepatectomy as well as liver transplant, patients with locally primary HB have a very good prognosis while those with metastatic disease have a very poor prognosis [4,27]. Therefore, approaches to suppress metastasis are the key to improving survival, the most important target for all treatments of HB patients as well as other types of cancer patients.
Metastasis is a process that tumor cells lose contact with other tumor cells in their primary foci, become mobile to invade neighboring tissues and basal membranes, enter bloodstream and/or lymphatic vessels and reach secondary sites in other organs [28]. EMT is widely considered a promoter of metastasis because such transformations enable tumor cells to acquire mobility, a mesenchymal feature closely associated with metastatic process [28]. β-catenin not only acts as a downstream effector of Wnt signaling pathway to activate transcription of key target genes responsible for cellular proliferation and differentiation but also directly mediates the interplay of adherent’s junction molecules with the actin cytoskeleton [29]. The cadherin switch, an event referring to the downregulation of E-cadherin and upregulation of N-cadherin, is closely associated with enhanced migratory and invasive traits of cancer cells [30]. Vimentin, an intermediate filament protein, acts as a positive regulator of EMT and is upregulation appears to be prerequisite of EMT [31].
MAP1S, originally named as C19ORF5, was firstly identified as an interactive protein of LRPPRC [32], its significance in tumorigenesis was initially suggested by its interaction with tumor suppressor RASSF1A [33], and its association with prognosis of patients with ovarian cancer was later revealed after analyzing a large database of human genetic mutation [34]. Reduced levels of MAP1S were found to be significantly associated with high malignancy and poor prognosis of patients with prostate adenocarcinomas and clear cell renal cell carcinomas [35,36]. Tumor suppressing role of MAP1S was experimentally confirmed in a mouse model of hepatocellular carcinomas [11-13]. Our results here demonstrated that, in consistent with results from other types of cancers, levels of MAP1S were significantly reduced in HB tissues and its downregulation of MAP1S was significantly associated with increased malignancies and metastasis and reduced survivals of HB patients. Suppressing levels of MAP1S in cultured HB cells led to increases in rates of cell proliferation, migration and invasion, suggesting an activation of EMT. Further tests revealed that levels β-catenin and mesenchymal markers N-cadherin and vimentin were elevated and those of epithelial marker E-cadherin were reduced after levels of MAP1S were reduced. It was clearly suggested that the downregulation of MAP1S in HB tissues may directly trigger the EMT of cancer cells and result in the promotion of metastasis and impairment of HB patients.
As a microtubule-associated protein, MAP1S was predicted to play important roles in the trafficking of organelles and chromosomes in cells [10,11,37-39]. Its specific interaction with autophagy marker LC3-II suggested a unique role in autophagy regulation and MAP1Smediated autophagy was found to be involved in the degradation of proteins such as fibronectin and mutant huntingtin and the suppression of oxidative stress, genome instability and tumorigenesis [10,13,18-20]. Here we found that suppressing the expression of MAP1S indeed blocks the degradation of mesenchymal markers N-cadherin and vimentin through autophagy. However, MAP1S also suppressed EMT through autophagy-independent mechanisms. Knockdown the expression of MAP1S in cultured cells led to the increases in mRNA levels of β-catenin and mesenchymal markers N-cadherin and vimentin and a reduction in levels of epithelial marker E-cadherin. The regulation of E-cadherin protein not through autophagy system may further confirmed previously published results that the stability of E-cadherin is mainly controlled by proteasome system, another major pathway of protein degradation [40]. As a microtubule-associated protein, MAP1S may impact the mRNA levels through either the regulating transcription of related genes or maintaining the stabilities of related mRNA molecules. We originally characterized that MAP1S had capacities to bind with DNA [41], which may suggest a potential mechanism of MAP1S to impact on mRNA levels. However, the exact mechanisms by which MAP1S regulates mRNA levels of those genes remain to be investigated.
In conclusion, similar to the situations in other types of cancers, MAP1S had reduced protein levels in HB tissues and MAP1Sdeficiencies were closely associated with enhanced metastasis and poor prognosis of the patients. Suppressing expression of MAP1S led to changes in expression levels of EMT-associated proteins through mechanisms of both autophagy-dependent and autophagy independent and increases in rates of cell proliferation, migration and invasion. Most of HB patients collected in the experiment had undergone chemotherapy which usually interferes autophagy flux through different mechanisms. Since we compared the differences between HB tissues and adjacent normal tissues, the impacts of chemotherapy on autophagy flux were supposed to be similar even if not exact between normal and HB tissues. The differences of autophagy impacts of chemotherapy between normal and HB tissues should mainly reflect the differences of MAP1S levels. Therefore, MAP1S may prevent metastasis and enhance survival of HB patients by suppressing EMT.
This study was supported by National Natural Science Foundation of China (NSFC) 81772931 to Leyuan Liu, Science and Technology Program of Guangzhou City of Guangdong Province of China 20210210128 to Xinke Zhou, Natural Science Foundation of Guangdong Province of China B2022148 to Boyun Shi and Key Laboratory of Guangdong Higher Education Institutes 2021KSYS009 to the Hospital.
Conflict of Interests
The authors declare no competing financial interests.
Author Contribution
BS, YW, ZY, WW, JH, KH, XZ and LL have full access to all of the data in the study, take responsibility for the integrity of the data and the accuracy of the data analysis and write the manuscript. MY and YZ contribute reagents and technical assistance.
Availability of Data and Materials
The data that support the findings of this study are available from the corresponding author on reasonable request.
Ethical Approval
An ethical approval for human subjects was obtained from the Research and Ethics Committees of The Fifth Affiliated Hospital of Guangzhou Medical University and Sun Yat-sen University Cancer Center. HB tissues and their adjacent normal tissues were collected from HB patients undergone curative liver resection in the hospitals with consent in writing to donate tissue specimens for the intended research from patients themselves or members of their families.


