Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Ogana Joy1, Orji Ejike Celestine1*, Nworji Ogochukwu Frances1 and Okechukwu NN Okoye2
Received: May 26, 2023; Published: June 06, 2023
*Corresponding author: Orji Ejike Celestine, Department of Applied Biochemistry, Nnamdi Azikiwe University, Awka, Anambra State, Nigeria
DOI: 10.26717/BJSTR.2023.50.008021
Heavy metals are known to cause deleterious effects on human health throughout the food chain. This study aimed to assess the heavy metals concentration in fish and potable water in Banegbe River and Ekpan Pond. Also, the Human health risks from the consumption of heavy metal-contaminated fish from the river were evaluated. To this aim, fish and water samples were collected at three different points in the river. Nine metals (cadmium Cd, iron Fe, arsenic As, manganese Mn, zinc Zn, mercury Hg, chromium Cr, copper Cu, and nickel, Ni) in the fish and water were analyzed using Atomic Absorption Spectrophotometer (AAS). The mean concentration values recorded in the water and fishes were in the range of 0.73±0.16- 2.85±0.18mg/kg for Cu 2.49±0.00 - 2.85±0.18mg/kg, for Zn 0.32±0.09-3.88±0.01mg/kg, for As 0.06±0.00 - 1.21±0.001mg/kg, for Cd 0.12±0.00 - 0.69±0.02mg/kg, for Cr 0.10±0.00- 0.62±0.00, for Pb 0.07±0.01- 0.34±0.02mg/kg, for Hg 0.72±0.24 1.99±0.05mg/kg, for Fe 0.23±0.01 - 0.47±0.01mg/kg, for Mn 3.22±0.01 and 0.82± 0.10. The results of this research showed that the concentrations of most of the metals at some points of sample collection exceeded the recommended limit set by WHO for potable drinking water except for Zn at all the sampled points. Assessment of non-carcinogenic health hazards by target hazard quotient (THQ) indicated no concern from consumption of these fish except for As, Hg, and Cd at middle-stream and Ekpan pond. However, all metals together may affect human health as revealed by the hazard index (HI). The target cancer risk (TR) values suggested carcinogenic risk from Pb, Cd, and As. Taken together it can be concluded that there is a potential human health risk in consuming fish from river Banegbe.
Keywords: Heavy Metals; Risk Assessment; Target Hazard Quotient (THQ); Hazard Index (HI) Banegbe River
Abbreviations: THQ: Target Hazard Quotient; HI: Hazard Index; TR: Target Cancer Risk; LCR: Life Time Cancer Risk; MS: Morbidity Status; EF: Enrichment Factor; AAS: Atomic Absorption Spectrophotometer; HRI: Health Risk Index; UF: Uptake/Transfer Factor
In Nigeria today, numerous studies indicated that industrial activities release heavy metals either as solids, gas, and most especially liquids in the form of wastewater or effluents allowed to drain into waterways or bodies. This pollution of the aquatic environment by organic and inorganic chemicals has been considered as a major threat to aquatic organisms including fish (Ali, et al. [1]). In recent years more attention has been paid to the contamination of the water ecosystem and their entry into the food chain (Ahmed, et al. [2]). Chemical contamination of food is considered the most significant source of human health risks. The major sources of foodborne diseases are microbiological and chemical hazards. Health risk due to the consumption of food from aquatic ecosystems contaminated with hazardous chemicals including metals has increased globally (Abu, Hilal, and Ismail, [3]), especially in developing countries such as Nigeria. The increasing usage of heavy metals in the industry has led to the increased release of harmful heavy metals into the aquatic environment (Agusa, et al. [4,5]). Heavy metals are naturally occurring elements that have a high atomic weight and a density at least 5 times greater than that of water. They enter the water bodies mainly through anthropogenic sources (Nadel, et al. [6]).
Their multiple industrial, domestic, agricultural, medical, and technological applications have led to their distribution in the environment; they get distributed in the aquatic body, sediments, and tended solids during the course of their mobility. Some of these metals are essential to living organisms and may become highly toxic when present in certain concentrations (Ahmed, et al. [7]). Some are also soluble in water and readily absorbed into living organisms. Metal ions of high toxicity are known to cause a deleterious impact on organs and blood levels in fish. They form metal complexes with the structural proteins, enzymes, and nucleic acids and interrupt their functions. Heavy metals are non-biodegradable having long biological half-lives (Heidarieh, et al. [8]). They also have the tendency to accumulate in various organs and muscle tissue of fish, raising concerns over their potential effects on human health and the environment. Their toxicity depends on several factors including the route of exposure, dose, and chemical species, as well as the age, gender, genetics, and nutritional status of the individual exposed. There is an increased risk of public exposure to heavy metals because of the consumption of aquatic food (like fish) from contaminated water (Ali, et al. [9]).
Fish is at the apex of the aquatic food chain, and during its life it can bioaccumulate large amounts of toxic elements (Nwabunike [10]). It is recognized that in freshwater systems, trace metals have high pollution potential that could be measured through the use of fish. The results of research with fishes indicate that metal distributions in fishes are both species-specific and site-specific. Fishes are an important part of the human diet in developing countries and are known as an essential component of a well-balanced diet worldwide because it provides carbohydrates, proteins, vitamins, essential fatty acids (like omega 3), minerals as well as trace elements (Pieniak, et al. [11]). Despite its nutritive value, the consumption of fish can cause potential hazard concerns for humans (Duffy and Zhang [12]). Fish assimilate metals by ingesting particulate material suspended in water, ingestion of food, ion exchange across lipophilic membranes (for example the gills), and adsorption on membrane and tissue surfaces. As such, fish may bioaccumulate large concentrations of metals from the water (Mansour and Sidky [13]) and the degree of accumulation rate is dependent both on uptake and elimination rates (Guven, et al. [14]). The age of the fish, lipid content in the tissue, and mode of feeding are significant factors that also affect the accumulation of heavy metals in fish. Fish have been reported in several studies as a source of heavy metals in humans through consumption (Castro-González and Méndez-Armenta [15]).
These metals can cause significant health risks to humans, particularly in elevated concentrations above the very low body requirement. Even at a very low concentration, heavy metals have serious toxic effects on animal and human populations (Aijire, et al. [16]). For example, Lead contaminant causes lipid peroxidation in tissues and causes irreversible damage to the respiratory organs of fish (Renier, et al. [17]). Nickel causes a morphological transformation and chromosomal aberration in cells, while cobalt induces convulsions, DNA strand breakage and organ damage. Also, hexavalent chromium is acutely toxic, mutagenic, teratogenic and carcinogenic to aquatic organisms and is relatively mobile in the environment (Alipour and Hassanpour [18]) Generally the excessive concentration of heavy metals in food causes a number of diseases especially cardiovascular, renal, neurological, mental impairment and bone diseases (Fu and Xi [19]). So heavy metals must be regulated in food sources in order to assure public health safety.
It has been indicated that dietary intake is the main route of exposure to heavy metals for most people, especially dietary intake of the aquatic organism like fish (Zhuang, et al. [20]). That is why fish has been shown as one of the most indicative factors in freshwater systems, for the estimation calculation or determination of trace metals pollution and risk potential of human consumption (Alhashemi, et al. [21,22]). The challenge of heavy metals entering the food chain needs a systematic assessment to make timely decisions to avert severe health effects because of the invisible way of heavy metal toxicity (Chary, et al. [23]). Assessments of risk have been done using various risk assessment techniques, such as statistics, geostatistics and geographic information systems GIS (Hani, et al. [24]), the Health Risk Index (HRI) (Khan, et al. [25]), the uptake/transfer factor (UF) (Tijani [26]), the morbidity status (MS) (Srinivasan and Reddy [27]), the enrichment factor (EF), the target hazard quotient (Chary, et al. [23]), the degree of contamination (Cdeg). In this work, target hazard quotient, hazard index and life time cancer risk (LCR) were used to assess the risks associated with the consumption of contaminated Papyrocraruns afer from Banegbe river.
Collection of Fish and Water Samples
Fish and water samples were collected from different stations (Figure 1) of the Banegbe river; Latitude 5o 14N and longitude 5o 22E, Latitude 5o 28N and longitude 5o 10E, and Latitude 5o 43N, longitude 5o 14E and latitude 5o 30N and longitude 5o 44E. Fishes were collected with the help of professional fishermen while they were fishing in the river. The fish samples were of similar size and weight. The samples were immediately preserved in air-sealed plastic bags and transported to the laboratory.
Note: Where P1= up-stream,
P2 = effluent discharge point,
P3 = down-stream,
P4 = Ekpan pond.
Digestion of Samples for Analysis
Wastewater samples (50 mL) were measured into a beaker, followed by the addition of 3ml concentrated HNO3 acid. For fish samples, 1g of fish muscles were put into 50 mL of HNO3. The mixture was boiled and evaporated in a steam bath to the lowest volume possible (< 5 mL) before precipitation occurs. The mixtures were allowed to cool, and then heated further with an additional 5ml of concentrated HNO3 and then in excess until digestion was completed as shown by a light-coloured clear solution. The samples were evaporated to less than 5mL and allowed to cool, 10mL (1:1) HCl and 15mL distilled water was added to the cooled sample and heated for additional 15 minutes to dissolve any precipitates or residue. The walls of the beakers were washed with distilled water and then filtered. The filtrates were transferred to a 100ml volumetric flask and diluted to mark with distilled water. Portions of the solution were taken for heavy metal analysis.
Analysis of Heavy Metals
The analysis of the digests was carried out by the use of an atomic absorption spectrophotometer (AAS) (ALPHA-4-AAS MODEL). The source of radiation was a hollow cathode lamp. The Atomic Absorption Spectrophotometer was calibrated for the different metals. Standard solutions of each metal salt and blank sample were analyzed with each set of digests.Statistical Analysis
Analysis of Variance (ANOVA) was used to analyze results.
Non-Carcinogenic Health hazard and Carcinogenic Risk Estimation
The target hazard quotient (THQ) assessed the non-carcinogenic health hazards for each individual metal through fish consumption, while the hazard index (HI) was estimated as the sum of the THQs (USEPA [28]). The THQ assumes a level of exposure (i.e., RfD) below which it is unlikely for even sensitive populations to experience adverse health effects. On the other hand, HI indicates the combined hazard of all metals. For carcinogens, Target risks (TR) were estimated as the incremental probability of an individual to develop cancer over a lifetime, as a result of exposure to that potential carcinogen (i.e., incremental or excess individual lifetime cancer risk (USEPA [29]). The THQ, HI and TR values were estimated using Eqs. (1), (2), and (3) respectively.

Where C is the concentration of individual metal in the fish, IR is the ingestion rate of the metal based on the average fish consumption rate set to be 0.0548kg /day/ person from the annual per capita fish consumption of 11kg or 9kg for Nigeria (FAO, 2008). BW is body weight which is set to be 70kg. AT is the averaging time = ED×EF where ED is the exposed duration set for 70 years life time EF the exposed frequency set for 7 times a week for the consumption of fish. With the RfD of Cu 0.04, Zn 0.3, Fe 0.7, Cr 0.003, Cd 0.001 As 0.0003, Hg 0.0003, Pb 0.004 Mn 0.14 and cancer slope factor of As 1.5, Pb 0.009 and Cd 0.6 (USEPA, 2011)
Hazard Index (HI) which is the sum of the individual hazard quotient of each metal
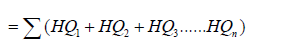
TR=Average Daily Dose × cancer slope factor……. (3)
Physicochemical Properties of Banegbe River at Different Locations (Up-Stream, Effluent Discharge Point, And Down-Stream) and Reference Point (Ekpan Pond Water)
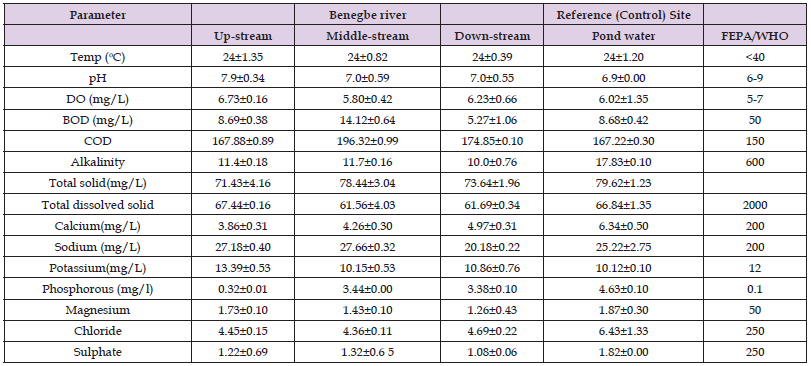
The result of the physicochemical properties of water samples from different points of Banegbe River and the control (Ekpan pond) revealed that the temperature, pH, dissolved oxygen, biological oxygen demand, alkalinity, and total dissolved solid were all within the WHO permissive limit for surfacse water bodies as shown in (Table 1), except for chemical oxygen demand that was above the WHO permissive limit as shown in (Table 1).
Table 1: Physiochemical properties of Banegbe river at different locations (up-stream, middle-stream and down-stream) and reference points (Ekpan pond water).
Note: All results are mean ± SD for 3 determinations
Heavy Metal Concentrations In, Banegbe River at Different Locations (Up-Stream, Middle-Stream and Down-Stream) and Reference Point (Ekpan Pond Water)
From the result presented in (Table 2), the metal contents of Ekpan pond water were significantly (P< 0.05) higher than Banegbe River. There was a significant (p<0.05) increase in most of the metal content at the middle stream when compared to the other points of study at Banegbe River. For the middle stream-ream of Banegbe river and down-stream, the order of metal increase was Zn > Fe > Cu >As > Hg > Cr > Cde except for a few variations, whereas up-stream followed the increasing order; Zn > Fe > Mn > Cu > As > Pb > Hg > Cd > Cr.
Table 2: Heavy metal concentrations of Banegbe river at different locations (up-stream, middle-stream and down-stream) and reference point (Ekpan pond water).

Heavy Metal Concentrations in Fish from Banegbe River at Different Locations (Up-Stream, Middle-Stream And Down-Stream) and Reference Point (Ekpan Pond Water)
The metal contents of the fish samples are shown in (Table 3). Results obtained revealed that fish samples harvested from Ekpan pond accumulated significantly (p<0.05) higher metals when compared to different locations at Banegbe River. Fish samples harvested from middle-stream of Benegbe River and down-stream accumulated higher metals content than up-stream.
Table 3: Heavy metal concentrations in fish from Banegbe River at different locations (up-stream, middle-stream and down-stream) and reference point (Ekpan pond water). All results are mean ± SD for 3 determinations.

Note: All results are mean ± SD for 3 determinations.
Target Hazard Quotient (Thq) For Individual Metal from Fish Consumption at Different Location of Banegbe River
From the calculated THQ and HI values, Ekpan pond has the highest followed by middle-stream, then down-stream and up-stream of Banegbe river. The increasing order of metals is as follow: As > Hg > Cr > Pb > Cu > Zn > Mn.
Estimated Target Cancer Risk (TR) for Individual Metal from Fish Consumption at Different Location of Banegbe River
From (Table 4) above the TR of Cd, Cr, and Pb up-stream middle-stream down-stream and Ekpan pond were within the USEPA standard of 10-4 to 10-6 while the TR of as was above the standard.
Table 4: Estimated Target Cancer Risk (TR) for individual metal from fish consumption at different location of Banegbe river.

The physiochemical parameters analysed at the different locations of Banegbe river were all within WHO and FEPA permissible limit for surface water, (FEPA/FMENV, [30]) except for chemical oxygen demand that were above the permissive limit of FEPA as shown in (Table 1). Middle-stream of Banegbe River had significant higher value of biological oxygen demand, chemical oxygen demand and total solid. The middle-stream of Banegbe River also had significant low value of demand oxygen when compared with other locations at the river, these may be attributed to pollution load from industrial effluents. (Akaninwor, et al. [31]) reported a high value of COD in polluted water samples which is similar to our findings. Although most of the parameters were within the permissive limit, a little variation in their value can affect the aquatic organism and absorption of pollutants by the aquatic organism in the river. For example, the temperature of the water body may lead to differences in metal deposition in various fish organs. Higher temperatures can lead to an increase in the accumulation of some metals in the kidneys and liver organs of fish (Yang, et al. [32]). The higher rate of metal uptake and binding of fish at higher temperatures may be a result of a higher metabolic rate (Grieb, et al. [33]). Also, different dates have indicated that water pH directly affects metal accumulation rates by the fish. In our present result upstream of Banegbe River has the highest pH concentration while Ekpan Pond had the lowest. Accumulation of copper is also higher at lower pH (Cogun and Kargin, [34]).
Banegbe is one the tributary of the Warri River, the river plays an important role for fisheries and support thousands of people for their bath and other domestic purpose Some of the industries located along the river empty their untreated effluent into the river. There was the presence of heavy metals (Cu, Zn, As, Cd, Cr, Pb, Hg, Fe, and Mn) in all the sampled point of the river. The Significantly high levels of most of the metals at middle-stream of Banegbe river as shown in (Table 2), were primarily as a result of pollution attributed to the discharge of untreated effluents from industries cited close to the course of the river which is channelled into the Banegbe river. Middle-stream was highly contaminated when compared to the rest of the sampling points. The level of the metals was observed to decrease from down- stream of Banegbe river, due to dilution, up-stream of Banegbe river had significantly lower concentration of these metals in all the metals analyzed when compared to middle-stream and down-stream of the river.The concentrations of some of the metals analyzed were found to be above permissible limits by WHO and FEPA (Table 2) for surface water bodies. These metals tend to bioaccumulate and are stored faster than excreted in aquatic organisms. Banegbe River serve as a source of drinking and cooking water for communities along the river especially fishermen, as such there is risk posed to the people of these communities since some of the toxic metals exceeded the safe drinking water limit by WHO (WHO [35]). However, the unexpected low values of some of these metals in our result at down-stream of Banegbe River when compared to middle-stream as shown in (Table 2) may be as a result of physical or chemical phenomenon such as mobility, adsorption or co-precipitation of metals or existence of some respiratory mechanisms by aquatic fauna to eliminate accumulated metal ions in the river.
Ekpan pond water had significantly higher concentration of these metals when compared to different locations at Banegbe River, and could be as a result of highly industrialization of Ekpan in Warri and the pond is not artificial one, but water is drained from Warri River to build the pond at the bank of Warri River in Ekpan. More over African kinfefish is not rear artificially. Similar to our result, is the report of (Ayenimo, et al. [36]) who showed that heavy metal content was higher at three different industrial locations of Warri River when compared to the other point along the river. (Egborge [37]) reported a base line of some heavy metals in Warri River which Banegbe river is a tributary, Cu was reported to have 9.2×10- 3, Fe (1.0×10- 5 – 4.0×10- 4 μgl-1), Cd (8.0 x 10-4 μgl-1), Pb (2.0x10- 3 μgl-1) Cr with the range of 169.25 – 235.17 μgl-1. Zn (2.0 x 10-3 – 0.006 x 10-3 μgl-1) that is far lower than our present result. (Ogbonna, et al. [38]) also reported the presences of some heavy metals in Ubayi River, with the middle-stream having higher concentration of the metals compared to up-stream and down-stream due to untreated effluents received from industries cited along the middle-stream of the river which is in agreement with our present findings with the middle-stream that receive effluent from different industries along Banegbe river having the highest concentrations of the metals. (Aghoghovwia, et al. [39]), also reported the presence of heavy metals at nine different locations in the Warri River, with the points of different industrial effluent discharge like steel companies having the highest values of these metals.
Comparing the results of our study with the above previous investigations at river Warri River in general, there is apparently an increase in the periodical accumulation of metals in the Banegbe River. This suggests that industrialization must have contributed a fair amount of these metals to the river. This may explain why there is a drastic reduction in marine organisms nowadays than preindustrial times.Fish is widely acceptable for consumption because of its high palatability, low cholesterol, and tender flesh (Eyo [40]), and also a cheap source of protein. The demand for freshwater fish can still be improved if information dissemination on the valuable nutritional composition of fish reaches the grass route. Besides this, there is the need to raise consumer awareness to ensure that contaminated fish are avoided. This will relieve the body of the burden of these toxic chemicals. The amount of contaminant in fish is dependent on the concentration level of these metals in food and their habitats as well as the detoxification rate of the metal. From our study, it was observed that heavy metal concentrations were significantly (p< 0.05) higher in fish samples when compared to the concentration in the water environment during the dry season. This is because fish like other aquatic organisms, have the capacity to bioaccumulate heavy metals in their tissues to levels that are higher than those in water, thus being able to elicit toxicological effects (Ravera, et al. [41]).
This could be the reason for the BAF of some of the metals being greater than 1 as shown in (Table 4). The bioaccumulation of metals by fish has been reported by other researchers including (Oguzie and Izevbigie [42]), whose report was on silver in catfish caught upstream of the Ikopba river and reservoir in Benin City. (Wangboje and Oronsaye [43]) also reported bioaccumulation of heavy metals in fish from Ogba River. The muscle of fish is the main part of human diet for most species throughout the world. So, in this present study the metal contents of the muscles were only analyzed being the edible part of the fish. Some researchers have reported high level of metals in the other tissue than the muscle tissue (Bervoets, et al. [44]). Though the concentration of some of these metals in fish muscle tissue are lower than WHO recommended permissive level in fish. Even at low concentrations of some of these metals can cause mental retardation, neurological, reproductive defects and physical disability in children. Higher levels can lead to coma, convulsions, and even death (Mahboob, et al. [45]). The THQ as a measure of health risk may signify some level of concern (Bassey, et al. [46]) THQ does not predict the actual adverse health effect on the exposed pollution but offer a signal of the risk level due to pollutant exposure. The acceptable guideline value for THQ is 1 (USEPA [47]). The Target hazard quotient (THQ) estimated for individual heavy metals through consumption of fish.
THQ values were less than 1 for all individual heavy metal except As, Hg and Cd at Ekpan pond and some sampled point in Banegbe river, which indicates non-carcinogenic potential health risk from ingestion of a single heavy metal through consumption of these fishes expect at the above-mentioned points. However Humans are often exposed to more than one pollutant and suffer combined or interactive effects (Li, et al. [48]). So, the combined impacts of all metals under consideration were higher than the acceptable limit of 1 for HI expect for up-stream. Sometime the HI might overestimate the potential for non-cancer health effects. This is because the toxicological effects associated with exposure to multiple chemicals, often through different exposure pathways, may not be additive (Table 5). Moreover, the effect of one metal is supposed to be dependent on the others due to the competitive absorption of metal ions in specific tissues of concern. The risk associated with the carcinogenic effects of target metal is expressed as the excess probability of contracting cancer over a lifetime of 70 years (Table 6). However, the THQ and HI are not direct measurements of risks because it does not define any dose–response relationship (USEPA, [28]). The TR values were estimated for the metals (Pb, A, s, and Cd) reported with known carcinogenic effects are presented in (Table 7). In general, the TR values lower than E−6 are considered to be negligible for carcinogenic risk, cancer risks lying between E−6 and E−4 are generally considered an acceptable range (Fryer, et al. [49]), and risk above E−06 are considered unacceptable (USEPA [29]). The TR values for Pb, As, and Cd in this study were all above E-06 as such can pose a carcinogenic risk. This observation is in agreement with (Adegbola, et al. [50]), who reported similar health risks for Cd and Ni in Clarias gariepinus and Sarotherodon from industrially polluted Ogun and Eleyele Rivers in Nigeria. Also, (Ahmed, et al. [51]) reported similar results for some other fish species collected from the river Buriganga.
Table 5: Estimated Daily intake for individual metal from fish consumption at different location of Banegbe river.
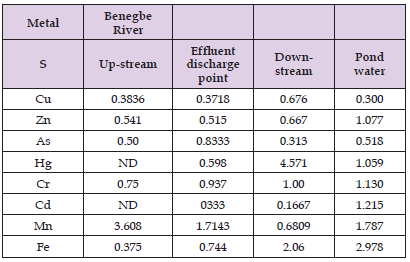
Table 6: Target harzard quotient (THQ) for individual metal from fish consumption at different location of Banegbe river.

Note: HI =Σ (HQl +HQ2 + HQ3……...HQn)
Conclusively from this study, long-time consumption of fish from Banegbe River might pose some health concerns suggesting that consumers of fish from the sites might experience non-carcinogenic health risks and carcinogenic risks also. As such the accumulation of some of these heavy metals raise concern and therefore requires a prompt response from necessary regulation agencies in the country.
Ogana J. conceived of the work and participated in the design and coordination of the work. Orji, E. C. participated in alignment and draft of the manuscript and also helped in results analysis and discussions. Nworji, O. F. participated in oversight and leadership responsibility for the work. Okoye, O. N. N. proofread, corrected, and aligned the manuscript. All authors read and approved the final manuscript.
Thanks to the Department of Biochemistry, University of Nigeria, Nsukka for providing the chemical reagents and equipment for this study.
The data are not publicly available due to privacy or ethical restrictions.
No funding was received for this work.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The study was conducted in accordance with the regulations and ethical approval of the Ethics and Biosafety Committee of the Faculty of Biological Sciences, University of Nigeria, Nsukka.


