Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
EV Husakouskaya*, NYe Maksimovich and HY Rathnamalala
Received: May 10, 2023; Published: June 02, 2023
*Corresponding author: E V Husakouskaya, Grodno State Medical University, Department of Pathophysiology named after D A Maslakov, Belarus, Grodno, Gorkogo Street, Belarus
DOI: 10.26717/BJSTR.2023.50.008012
Background: The slow tendency to decrease in mortality in peritonitis may be due to incomplete
understanding of its pathogenesis.
Objective: To study some indicators of intoxication and leukocyte response in rodent fecal peritonitis.
Material and Methods: The experiments were carried out on male rats (n=74), divided into 2 equal
series, which were injected intraperitoneally with: 1st series (control) – 0,9% sodium chloride, 2nd series
(experimental peritonitis, EP) – 15 % fecal suspension, 0,6 ml/100 g. Studies were made after half a day
(n=6), one day (n=6) and three days (n=6) of EP, lethality of animals also was assessed (n=19). In rats
with fecal peritonitis, some indicators of intoxication were studied by assessing of motor activity, muscle
strength, respiration rate and rectal temperature, and the reaction of blood and peritoneal leukocytes
basing on determining their quantitative composition and phagocytic activity.
Results: In rats with EP, a decrease in motor activity and muscle strength, the development of fever and
tachypnea along with changes in blood and peritoneal fluid in type of leukocytosis, an increase in content
of neutrophils and macrophages, the appearance of metamyelocytes and myelocytes, a decrease in content
of peritoneal formazan-positive neutrophils and blood lymphocytes, absence of eosinophils were found.
Conclusion: The study of changes in rats with acute experimental peritonitis revealed the development
of severe intoxication, neutrophil-macrophage leukocytosis with a hyperregenerative shift to the left and
a decrease in the phagocytic activity of neutrophils along with lymphopenia and absence of eosinophils.
Keywords: Peritonitis; Intoxication; Leukocytes; Phagocytosis; Rodents
Diffuse peritonitis remains one of the urgent problems of surgery due to the persistent high mortality rate of 27,8-53,4 %, which reaches 85-90% with the development of septic shock and multiple organ failure [1-3]. At the same time, the most numerous group of patients with peritonitis is a working age group [4], and its treatment requires significant economic expenses, which are much more higher than the expenses for treatment of diseases not accompanied by infectious complications [5]. These data indicate the significant socioeconomic significance of peritonitis. Surgery is an obligatory standard in the treatment of diffuse peritonitis and is always combined with antibiotic therapy, which is quite well developed [6]. However, a high mortality may be due to the inferiority of the pathogenetic therapy of peritonitis due to insufficient understanding of the mechanisms of its development. Until now, the mechanisms of the leukocyte’s migration and regulation of their activity in peritonitis remain incompletely studied, while the influence on them can improve the prediction in the pathology. The objective of the study was to study some indicators of intoxication and leukocyte response in rodent fecal peritonitis.
Experiments were carried out on outbred male rats, 230-250 g (n=74), in accordance with the Helsinki Declaration on the Humane Treatment of Animals (1964). Rats were divided into 2 equal series, which were injected intraperitoneally with: 1st series (control) – 0,9% sodium chloride, 2 series (experimental peritonitis, EP) – 15 % fecal suspension, 0,6 ml/100 g of body weight, according to the modified method described by (VA Lazarenko, et al. [7,8]). The suspension was standardized according to the extinction coefficient and the number of bacterial cells [8]. In each series, studies on the severity of intoxication, changes in the quantitative composition of blood and peritoneal leukocytes and their functional activity were carried out after half a day (n=6), one day (n=6) and three days (n=6) after peritonitis modeling along with assessment of rats lethality (n=19). The rats motor activity was estimated after measuring of the distance traveled in the «open field» test, muscle strength – by keeping track of time of animals hold on to the mesh wire [9,10], respiratory rate – by counting the number of chest excursions per 1 minute, rectal temperature – measuring with an Omron ETS electronic thermometer [10]. The study of the quantitative composition of leukocytes was carried out in the Goryaev chamber and in blood smears and peritoneal fluid, stained with azure-eosin [11]. Determination of the ability of peritoneal neutrophils to phagocytosis was carried out on the basis of the percentage of formazan-positive neutrophils, using the adapted method of YuI Patsula, VS Vlasenko [12,13]. Statistical data processing was carried out in Statistica 10.0 program for Windows (StatSoft Inc., USA) using the nonparametric Kruskal-Wallis test and post hoc comparisons by Dunn’s test; data are presented as Me (LQ; UQ), where Me is the median, LQ and UQ are the values of the lower and upper quartiles, respectively.
The course of acute EP in rats was accompanied by the development of a pronounced intoxication and was manifested by severe general state, inhibition of motor activity, decrease in muscle strength, development of tachypnea and fever and high mortality (Table 1). In rats with acute fecal EP, a severe general state was noted, as evidenced by limitation of mobility, refusal to eat, polydipsia, absence of defecations against the background of an enlarge of the abdomen and tension of abdominal wall, which persisted until the death of some of animals. A decrease in motor activity was manifested as a decrease in the distance traveled by rats with EP in the «open field» test, after half a day – by 69,0 % (p<0,001), after 1 day – by 80,1 % (p<0,001), after 3 days – by 73,7 % (p<0,001). At the same time, the distance traveled after 1 day was less than after half a day by 35,9 % (p<0.05), indicating that the condition of the animals worsened over time. Along with a decrease in the motor activity of animals, a decrease in their muscle strength was noted, as indicated by a decrease in the time they were kept on the mesh wire after 0,5 days, 1 day and 3 days of EP – by 77,8 % (p<0,001), 87,0 % (p<0.001) and 83,3 % (p<0,001) respectively. Moreover, the time of rats keeping on the mesh wire after 1 day decreased by 41,5 % (p<0,05), compared with the value after half a day, confirming the aggravation of the intoxication. Inhibition of motor activity and decrease in muscle strength in rats with EP may be due to the high intensity of the inflammatory process and degree of intoxication, leading to metabolic disorders in muscle tissue and energy deficiency [14].
Note: *significant differences relative to: * – p<0,05, ** – p<0,01, *** – p<0,001 – control group; Ψ - p<0,05 – half a day and Δ – p<0,05 – 1 day within the group.
The breath rate in rats with EP increased after half a day – by 50,0 % (p<0.01), after 1 day – by 58,0 % (p<0,01), after 3 days – by 37,2 % (p<0.01), with the most pronounced tachypnea after 1 day of inflammation, while the respiratory rate was greater than after 0,5 days and 3 days by 5,7 % (p<0,05) and 15,5 % (p<0.05) respectively. The occurrence of tachypnea in rats with EP is due to the development of hypoxia and acidosis [15]. The development of fever in rats with EP was manifested by an increase in rectal temperature after half a day – by 2,6 (2,6; 2,7) °C, p<0,01, after 1 day – by 3,3 (3,3; 3,5) °C, p<0,01, after 3 days – by 1,6 (1,6; 1.8) °C, p<0,01, compared with the value in the «control» group, characterizing the development of pyretic fever on the first day and febrile – after 3 days of peritonitis. At the same time, the rectal temperature in rats after 1 day was higher than after half a day by 0,7 (0,6; 0,8) °C (p<0,05), and after 3 days – less than after half a day, by 1,0 (0,9; 1,0) °C (p<0,05) and 1,7 (1,6; 1.8) °C less than after 1 day (p<0,05). The lethality of animals with EP was 68,4%, which indicates the severity of the inflammatory process and intoxication. Thus, the course of acute fecal EP was accompanied by the development of a pronounced intoxication, to the greatest extent after 1 day of peritonitis, and was manifested by severe systemic condition, inhibition of motor activity, decrease in muscle strength, development of tachypnea and fever and high lethality of experimental animals. The total count, composition of blood and peritoneal leukocytes, phagocytic activity of peritoneal neutrophils of rats with EP were studied.
The quantitative composition of blood and peritoneal leukocytes of rats in the control group was characterized by a predominance of lymphocytes, a less numerous population was represented by segmented neutrophils and monocytes/macrophages, while eosinophils, basophils/mast cells were found in smaller quantity (Figure 1). When assessing the content of neutrophils in all the studied periods, an increase in the number of segmented and band neutrophils was noted along with the appearance of metamyelocytes. In addition, after one day and three days, myelocytes were found in the blood and peritoneal fluid of rats with EP, which characterizes the transformation of the regenerative shift of leukocyte differential count to the left into hyperregenerative one (Table 2). Along with a change in quantitative composition of neutrophils, a decrease in their ability to phagocytosis was noted. This was evidenced by a decrease in the percentage of formazan-positive neutrophils in the peritoneal fluid in half a day – up to 44 (42; 46) %, or by 13 % (p<0,05), one day – up to 35 (33; 38) %, or by 22 % (p<0,05), three days – up to 44 (41; 45) %, or by 13 % (p<0,05), (Figure 2). The study of the content of basophils/mast cells and eosinophils showed an increase in their number in the peritoneal fluid in all the studied periods, while in the blood in 0,5 days and 1 day of EP, basophils and eosinophils were not detected. At the same time, the reaction from both macrophages and lymphocytes of the peritoneal fluid was manifested as an increase in their content.
Table 2. The content of various types of leukocytes (x 106/l) in blood and peritoneal fluid of rats with experimental peritonitis (EP), Me (LQ; UQ).
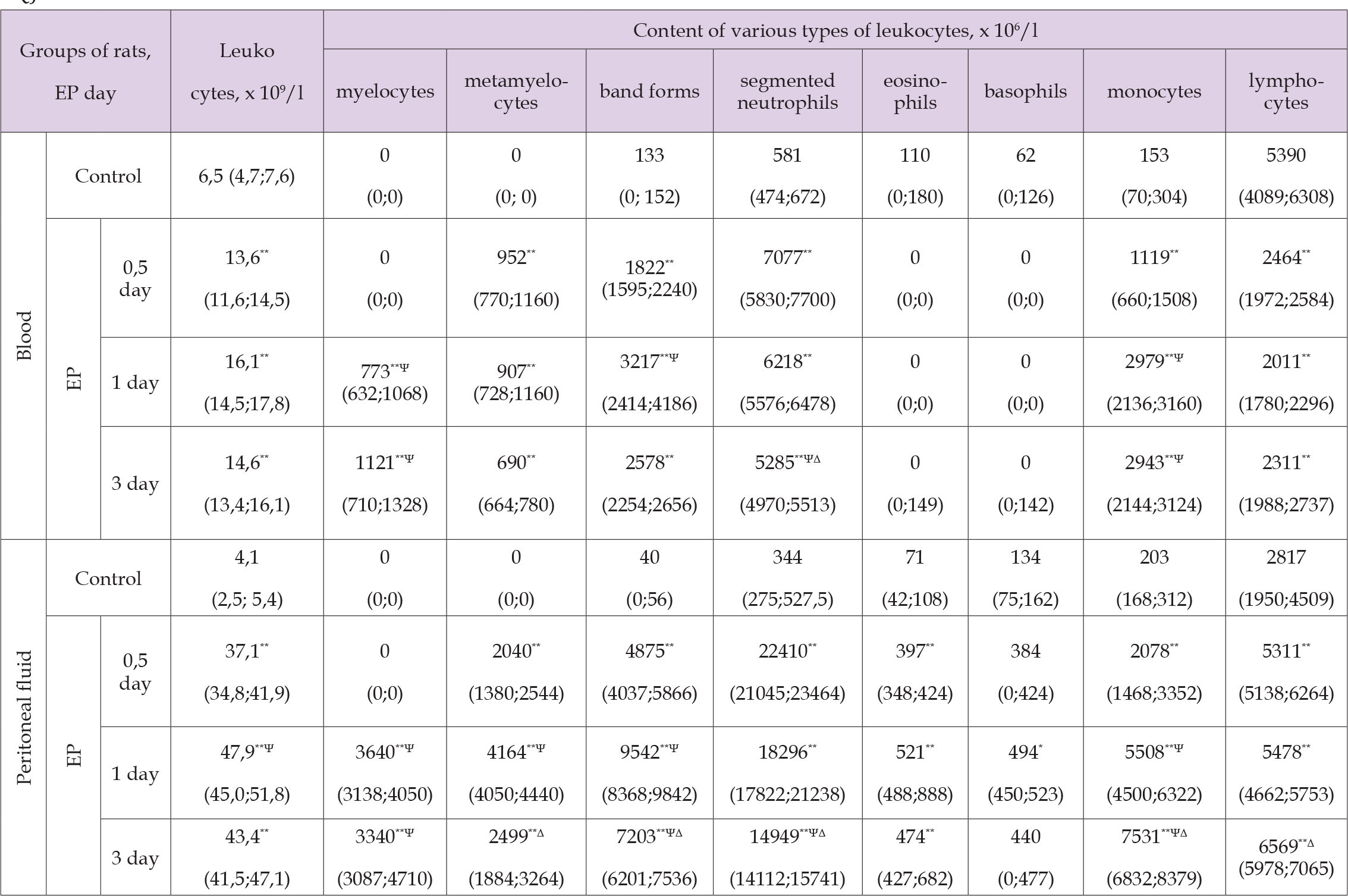
Thus, changes in the quantitative composition of blood and peritoneal leukocytes in rats with acute EP were characterized by the development of lymphopenia, absence of eosinophils and pronounced neutrophilic leukocytosis with a hyper regenerative shift of leukocyte differential count to the left and decrease in the ability of neutrophils to carry out phagocytosis. Thus, the studies of changes in rats with EP revealed the presence of significant disorders, which were expressed in a decrease in motor activity and muscle strength, the occurrence of tachypnea and fever, the development of neutrophilic-macrophage leukocytosis with a hyperregenerative shift of the leukocyte differential count to the left in blood and peritoneal fluid with a decrease in the ability of neutrophils to phagocytosis, lymphopenia and absence of esinophilis. Obtained data are valuable because they describe assessment of the intoxication activity and state of immune system. In addition, there are conflicting literature data on the functional activity of phagocytes, according to which its decrease or increase is noted [16]. The revealed decrease in motor activity and muscle strength in rats with EP may be due to high intensity of the inflammatory process in abdominal cavity and degree of intoxication, leading to muscle protein catabolism, energy deficiency, acidosis with an increase in membrane permeability, swelling of myocytes and a decrease in their contractility [16]. The occurrence of tachypnea may be a consequence of the hypoxia and acidosis, while violation of thermoregulation in the form of pyretic fever is associated with the production of secondary pyrogens (cytokines) and the PgE2 formation with a shift of the hypothalamic temperature set point [17].
The severity of inflammatory process and intoxication is evidenced by the high lethality of rats. A significant increase in the content of leukocytes in peritoneal fluid and less pronounced – in blood can reflect the activation of their exit from the «depot» and leukocytes migration into abdominal cavity. The development of neutrophilic leukocytosis indicates the active participation of neutrophils in the implementation of protective reactions in abdominal cavity, and the hyperregenerative shift of the leukocyte differential count to the left indicates a significant intensity of the infectious-inflammatory process [17]. The change in the functioning of immunocompetent cells was expressed in a decrease in the ability of neutrophils to phagocytosis, which may be due to the cytotoxic effect of reactive oxygen and nitrogen species on peritoneal microphages. An increase in the number of eosinophils and mast cells in the peritoneal fluid may be due to an increase in the transition of eosinophils and basophils from the bloodstream into abdominal cavity, where mast cells release biologically active substances, leading to an increase in vascular permeability, and eosinophils inhibit excessive mast cell degranulation. An increase in the content of macrophages and lymphocytes in the peritoneal fluid may be explained as a result of their migration from the bloodstream into abdominal cavity and is associated with the performed functions: phagocytosis – carried out by macrophages along with regulation of the immune response by lymphocytes [16].
The conducted results on the study of experimental peritonitis allow for a comprehensive assessment of changes in the body of rats and make a significant contribution to clarifying the mechanisms of pathology development. The obtained data indicate an obvious disruption in the functioning of immunocompetent cells and microcirculation system. The results of experiments can serve as a fundamental basis for further research, including practical medicine, in order to find effective approaches to correct the inflammatory process in abdominal cavity.


