Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Drita Abazi Bajrami1*, Mirko Marinkovski2, Kiril Lisichkov2 and Stefan Kuvendziev2
Received: May 13, 2023; Published: May 30, 2023
*Corresponding author: Drita Abazi Bajrami, Faculty of Technological Sciences, Mother Teresa University, Skopje, Republic of North Macedonia
DOI: 10.26717/BJSTR.2023.50.007994
In this paper, the kinetics of bioactive components from Helichrysum arenarium under ultrasounds action was investigated. The aim of this study was to do model validation by plotting experimental data and to be seen if the correlation coefficient fits the experimental data. The process takes place in two stages: a fast one in the first stage 0–75 min, followed by slow phase from 75-180 min, in the second phase the equilibrium concentration was approached after 80 min. The modelling of kinetics in two phases (fast and slow) is done through the mathematical interpretations of the kinetically controlled process and the diffusion controlled process, also with a separate analysis of the two phases separately and finally they are combined into one process. From the results it can be seen that in the first stage the correlation coefficient (R2) has values above 0.9, which indicates that the selected models perfectly fit the experimental data, while for the second stage, the correlation coefficients vary below 0.9 however, these values (above 0.8) show that this model is accurate enough to determine the kinetics of the extraction process. Based on the results, it can be concluded that this technique is suitable for obtaining bioactive substances.
Keywords: Bioactive Component; H. Arenarium; Extraction; Ethanol; Ultrasound
Ultrasound assisted extraction has very wide range of use, it can be used in pharmaceutical, cosmetic, and food industries, this technique is very effective, safe and environmentally friendly [1,2]. Physical and chemical effects, as well the cavtation are the base of ultrasound-assisted extraction [2]. When ultrasound passes through a liquid, the extension cycles use negative pressure on the liquid, pulling the molecules apart. The expansion cycle can generate cavitation bubbles in the liquid if the ultrasound intensity and pressure are sufficient. Depending on the type of liquid and its purity, the excess of negative pressure can also change local fluid drag force. The bubbles will absorb the energy from the sound waves and will grow during the expansion cycles and recompress during the compression cycle. During compression, the bubbles collapse due to the extremely high pressure and temperature. This will cause shock waves through the solvent, increasing the mass transfer within the system [3-8]. Ultrasound assisted extraction of bioactive compounds from different plants has been widely used due to the potential of this technique. The extracted bioactive compounds are an excellent natural source of antioxidants and bio-based products extracted at lower temperature [9-11]. To describe the kinetics and mechanism of extraction processes have been developed various scientific researches [4,9,12-15]. The behaviour of the extraction process can be predicted, controlled and evaluated with various mathematical models, those can contribute for the use of energy, time and solvent [16,17].
There are different modelling strategies, such as kinetic models and empirical models, these models help to better understand the parameters involved in the ultrasound extraction process [18]. The extraction of bioactive compounds is assumed to be controlled by two-phase boundaries. The first phase indicates for the washing process that the compounds are dissolved in the solvent, while the second phase is calculated as a slow phase due to mass transfer limitations, unlike the first phase. In the second phase, which is considered as a diffusion process, the dissolved substances from the cells of the inner wall are distributed in the solvent [16,19]. Experimental data are generally processed and analyzed using physical kinetic models to investigate the kinetics of the extraction process and to describe the mechanisms that drive it. Many kinetic studies have been developed to describe the process of ultrasoundassisted extraction of bioactive compounds from biomass and to achieve technological transfer from experimentally to medicinal and pharmaceutical uses [9]. Kinetic studies play an important role in the extraction process and extraction rates such as if the process is fast or slow. Through kinetic studies, different factors can be understood that affect the rate of the extraction process. There are a large number of studies on the ultrasound extraction process, but the number of studies on the kinetic aspect of H. arenarium is small. This study deals with experimental investigation of bioactive compounds from H. arenarium using solvents with different polarity and in different temperatures. The main goal is to evaluate if this kinetic model could be applied in order to assess optimum performance of the extraction process.
Helichrysum Arenarium has been used as raw material for the extraction of bioactive components. This material was bought in a pharmacy, produced by Alkaloid AD in Skopje.
Solvents
The solvents used are different, such as ethanol (96%, 70% and 55%), methanol, petroleum ether, n-hexane and methylene chloride produced by different companies, but all solvents were of a high level of analytical purity.
Extraction Equipment and Procedures
The ultrasound extraction took place in an ultrasonic bath of 30 l, generator with a power of 240 W and a frequency of 40 kHz. Based on the optimal working parameters achieved by the optimization process, the rate of extraction of Helichrysum arenarium oil was determined at three different temperatures, namely at temperatures of 20, 30 and 40°C. The extraction was carried out in several cycles starting from 2-180 min. In each cycle, 1 g of the plant was used as extraction material and 50 ml of solvent. At the end of each cycle, the extracts were filtered through simple filter paper and the amount of extracted oil was determined by dividing the oil and solvents using a rotary vacuum evaporator. The samples were stored in a dark room at 4⁰C.
Mathematical Modelling
In this study, the mathematical model was applied to model the kinetics of H. arenarium extraction, namely, two-phase kinetics modelling (kinetic and diffusion-controlled process). This model provides excellent results for modelling the kinetics of ultrasound assisted extraction. Kinetics in the extraction process take place in two phases, in this way the extraction process is divided into fast and slow phases. The results are interpreted as a dependence on the yields (Y) in relation to the extraction time (t). The results are divided into two phases, because the slope of the line in the first phase is significantly greater than the slope of the line in the second phase. This modelling model can use linear equations of the type:
y = a ' x + b
The regression coefficients are represented by the variables a and b, while the extraction time expressed in minutes is represented by x. MATLAB R2013a software was used to determine the regression coefficients using the Curve Fitting Toolbox command.
Kinetics of ultrasound assisted extraction was studied at different temperatures as shown in Figures below. Experiments were carried out under optimal reaction conditions, namely using different solvents such as 96% ethanol, 70% ethanol, 55% ethanol, petroleum ether, methanol and methylene chloride.
Modelling in Two Phases (Fast and Slow)
Modelling of the kinetics in two phases (fast and slow) is done through the mathematical interpretation of the kinetically controlled process and diffusion controlled process, also with special analysis of the two phases separately and finally they are combined into one process. In the Figure 1 are given the modelling of 96% ethanol, 70% ethanol, 55% ethanol, petroleum ether, methanol and methylene chloride in two phases at different temperatures.
Summarized Results for All Solvents in Both Extraction Stages
From Tables 1-3 it can be seen that in the first stage the correlation coefficient (R2) has values above 0.9, which shows that the selected models perfectly fit the experimental data, while for the second stage, the correlation coefficients vary below 0.9 however, those values (above 0.8) indicate that this model is sufficiently accurate to determine the kinetics of the extraction process. The results shown in Table 4 in the first stage are similar to the results shown in Tables 1 & 2, while in the second stage the results shown are different from the results in Tables 1 & 2. In the second stage, the correlation coefficients have values below 0.7. The results shown in Tables 5 & 6 show that the correlation coefficients have values above 0.9 in both phases (fast and slow), which indicates that the selected models fit the experimental data very well.
Table 1. Values of the coefficients in the fast and slow phase when 96% ethanol was used as a solvent for the ultrasound assisted extraction of Helichrysum arenarium.
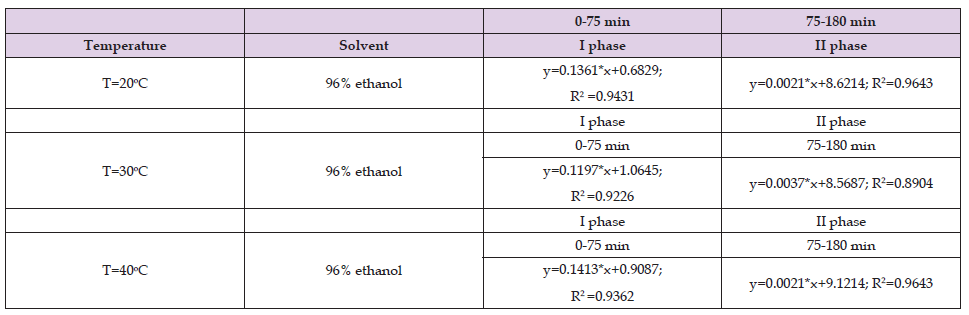
Table 2. Values of the coefficients in the fast and slow phase when 70% ethanol was used as a solvent for the ultrasound assisted extraction of Helichrysum arenarium.
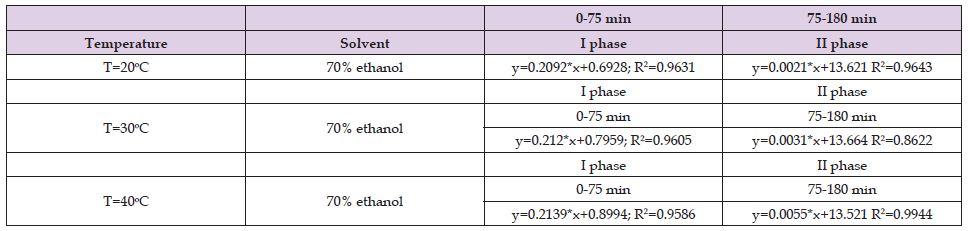
Table 3. Values of the coefficients in the fast and slow phase when petroleum ether was used as a solvent for the ultrasound assisted extraction of Helichrysum arenarium.
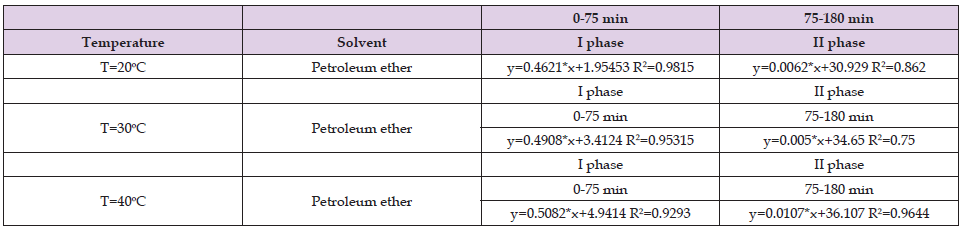
Table 4. Values of the coefficients in the fast and slow phase when 55% ethanol was used as a solvent for the ultrasound assisted extraction of Helichrysum arenarium.
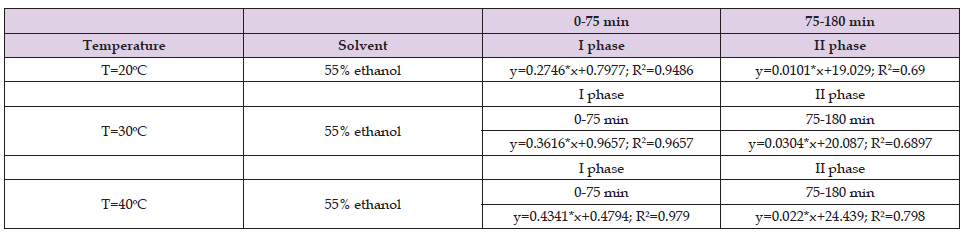
Table 5. Values of the coefficients in the fast and slow phase when methanol was used as a solvent for the ultrasound assisted extraction of Helichrysum arenarium.
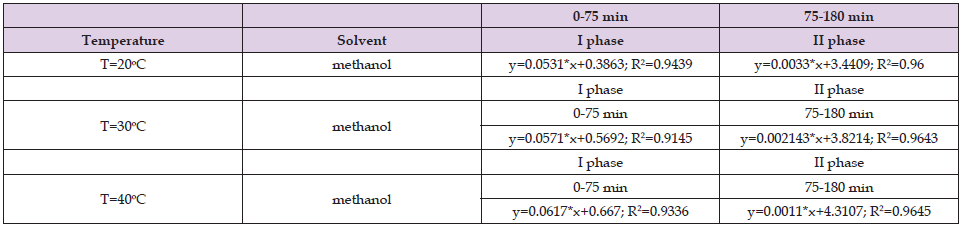
Table 6. Values of the coefficients in the fast and slow phase when methylene chlorid was used as a solvent for the ultrasound assisted extraction of Helichrysum arenarium.
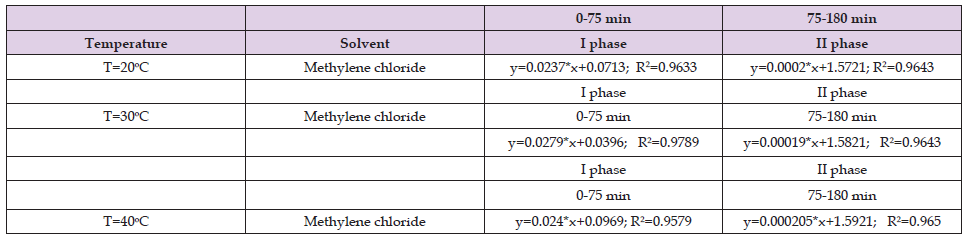
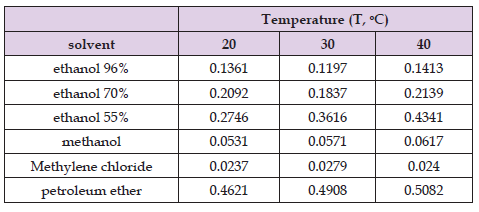
Table 7. Given values of slopes in the fast phase for ultrasound assisted extraction of H. arenarium for different temperatures.
Representation of Coefficients in Fast (I phase) and Slow Phases (II phase)
I phase (Fast Phase): From Table 7, Figure 2 it can be seen that with increasing extraction temperature, the slope values increase when 55% ethanol, methanol and petroleum ether were used as a solvents, while for the other solvents (96% ethanol, 70% ethanol ethanol and methyene chloride) it can vary. In 96% ethanol, with the increasing of the temperature to 30°C, the yield decreases, while increasing the temperature to 40°C, the yield increases. The same effect is achieved with 70% ethanol when it was used as a solvent, while with methylene chloride, the opposite effect is obtained from the previous two, i.e. with an increase in temperature to 30°C, the yield increases, and a further increase in temperature to 40°C contributes to a decrease in the total yield. The figure shows the slopes in the fast phase for different solvents at different temperatures.
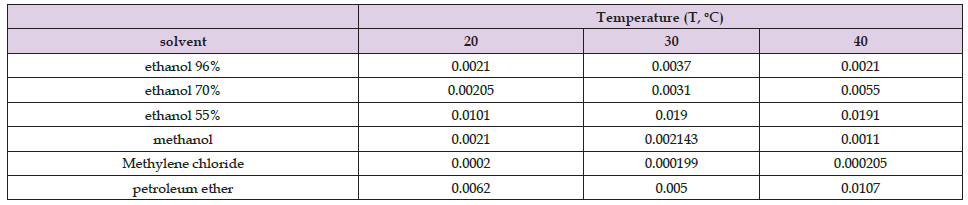
II phase (Slow Phase): Table 8 shows the values of the slopes in the slow phase for ultrasound assisted extraction of H. arenarium for different temperatures From the Table 8, Figure 3 it can be seen that with increasing extraction temperature, the slope values increase when 70% ethanol and 55% ethanol were used as a solvents, while for the other solvents (96%, methanol, methane chloride and petroleum ether) it varies. In 96% ethanol, increasing the temperature to 30°C, the yield increases, while increasing the temperature to 40°C, the yield decreases. The same effect is achieved with methanol when used as a solvent. In the case of methylene chloride as a solvent, from the figure and the table it can be seen that the increase in temperature has no significant effect because the slopes can be said to be constant, while in the case of petroleum ether, with an increase in temperature to 30⁰C the slope decreases, while as the temperature increases to 40⁰C, the slope increases. Figure 3 shows the slow phase slopes for ultrasound assisted extraction of H. arenarium with different solvents at different temperatures. In general, it can be concluded that the values of the slopes in the fast phase are much higher than the values of the slopes in the slow phase for all the solvents used in the ultrasound assisted extraction of H. arenarium. This phenomenon is expected because at the beginning the solvent is clean (unsaturated) and rapidly removes the soluble bio components from the raw material, while after approximately 1 hour, the solvents become saturated and can dissolve less bioactive components from the active raw material until the equilibrium of the systems. The individual comparison for each solvent in both phases is given in the figures that follow (Figure 4).
Table 8. The values of the slopes in the slow phase for ultrasound assisted extraction of H. arenarium for different temperatures.
Kinetic extraction study of bioactive components from H. arenarium in ultrasound assisted extraction in different period of extraction and temperature was experimentally investigated. The kinetics of extraction was developed based on the assumption of a two-phase kinetics modelling and kinetic parameters, recording a regression coefficient value of R2>0.98. The obtained results confirm that this technique can be used to increase the extraction performance of bioactive compounds, where the main factors are temperature and extraction time. Overall, all the obtained results can provide useful information for designing and optimizing the ultrasound-assisted extraction process.


