Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Jesús Alonso Gándara-Mireles1,2, Ismael Lares-Asseff1,2*, Elio Aarón Reyes Espinoza3, Verónica Loera Castañeda1,2, Julio Cesar Grijalva Avila1,2, Ignacio Villanueva Fierro1,2, María Fernanda Monroy Baroja1,2, Jose Luis Molina Marín1,2, Leslie Patrón-Romero4 and Horacio Almanza-Reyes2,4
Received: May 18, 2023; Published: May 30, 2023
*Corresponding author: Ismael Lares-Asseff, Academia de Genómica, Instituto Politécnico Nacional, CIIDIR-Unidad Durango, Red Latinoamericana de Implementación y Validación de Guías Clínicas Farmacogenómicas (RELIVAF-CYTED), México
DOI: 10.26717/BJSTR.2023.50.007993
We will be describing our experience with five patients diagnosed with acute lymphoblastic leukemia (ALL), who developed L-Asparaginase (L-Asp)-induced pancreatitis during their remission induction stage. The patients were treated, at the State Cancer Center of Durango, Mexico (CECAN-Durango) since December 2021, along with a group of 30 pediatric patients (in the remission induction phase of their treatment) who did not develop pancreatitis. All patients were diagnosed with (ALL) according to criteria from the French American-British Hematology Association (FAB). Once diagnosed, patients were treated according to the St. Jude Total XV protocol. In the protocol group, L-Asp enzyme activity and asparagine (Asp) concentrations were measured on 3 occasions. Population pharmacokinetic/pharmacodynamic (PK/PD) modeling using the previously assembled and validated monolix suite software was used to determine PK/PD parameters. Four of the patients after the ninth administration and one after the sixth administration of L-Asp revealed an increase in amylase and lipase enzymes even though the patients were asymptomatic. Three of the patients had abdominal distension and diffused pain. A computed axial tomography (CAT) scan confirmed the diagnosis of pancreatitis due to the use of L-Asp, which was immediately discontinued. Regarding Asp levels, it was observed that the five patients who developed pancreatitis reached complete depletion earlier than the 30 patients without pancreatitis. By analyzing the decay slope of Asp, we found that this could be a risk. marker for the development of pancreatitis as an adverse effect of the administration of L-Asp in patients with ALL.
Keywords: Asparagine; Ppancreatitis; Leukemia; Depletion
Abbreviations: FAB: Franco-American-British Association Of Hematology; CAT: A Computed Axial Tomography; SNP: Single Nucleotide Polymorphisms; AUC: Area Under The Curve; CSF: Cerebrospinal Fluid; ALL: Acute Lymphoblastic Leukemia
The administration of the enzyme L-Asparaginase (L-Asp) is the mainstay in the treatment of patients with acute lymphoblastic leukemia (ALL) [1]. The objective of its administration is to reduce the concentration of Asparagine (Asp) in both plasma and cerebrospinal fluid (CSF), leaving therefore the tumor cells without basic nutrients, for protein synthesis and causing cell death [2]. Although L-Asp is a cornerstone of ALL treatment, it is commonly associated with adverse effects, including pancreatitis [3-5]. In severe cases, it requires discontinuation of L-Asp treatment, which secondarily increases the risk of relapse in ALL patients [6]. Although pancreatitis is one of the common causes of intolerance to L-Asp, the reality is that the mechanism by which it is generated is unknown [7,8]. Recent protocols in the treatment of ALL, have increased the overall total doses of L-Asp treatment in selected high-risk populations, and have incorporated extended half-life formulations in order to reduce the number of patients who develop pancreatitis [6]. In addition, L-Asp monitoring strategies have been incorporated into the treatment, in the quest to ensure adequate levels of Asp depletion throughout treatment. Some studies have also included pharmacogenomic studies, such as single nucleotide polymorphisms (SNP), which in theory may identify patients at increased risk of pancreatitis, however, it is currently unknown whether these SNP, will effectively decrease the risk of pancreatitis. We describe five cases of L-Asp-induced pancreatitis, in five children aged 7 to 13 years, treated at the State Cancer Center of Durango, Mexico (CECAN-Durango) since December 2021, whom in common, they all had a rapid decrease in Asp levels. This could be a risk marker for developing pancreatitis secondary to L-Asp administration.
For the purposes of this study, and to determine the relationship between pharmacokinetics/ pharmacodynamics (PK/PD) of L-Asp and Asp levels, an analytical method was developed, and validated by spectrophotometry, in order to quantify the enzymatic activity of L-Asp, as well as Asp concentrations in blood. To perform this analysis, the Asparaginase Activity Assay Kit (MAK007, SIGMA, Saint Louis, MO 63103, USA) was used [9]. In addition, Asparagine Assay Kit (Fluorometric) (Sigma-Aldrich, MAK055) was used [10]. Monolix® software (version 2021R2. Antony, France: Lixoft) was used to determine the PK/PD parameters of L-Asp, using a previously assembled and validated population PK/PD model. This assay was performed with strict on-site instructions, and monitoring of blood sample processing, to minimize ex vivo hydrolysis of asparagine, including sample placement on ice, within 1 min of sample collection, centrifugation at 4°C, subsequent separation of plasma and storage at -70°C. For subsequent analysis.
Information from five patients is presented. Five pediatric patients: Two male and three female, between 7 and 13 years of age, Mexican, with no relevant family and/or medical history. They were admitted to the pediatric Hemato-Oncology service of CECAN-Durango, Mexico; after the diagnosis of ALL according to the criteria established by the Franco-American-British Association of Hematology (FAB) [11]. All began treatment under the protocol, St. Jude TOTAL XV [12], which includes the administration of 10,000 U/I m2 of L-Asparaginase (L-Asp) in the remission induction stage. Due to the history of side effects, such as pancreatitis, all patients were routinely monitored to determine pancreatic lipase and amylase enzymes, in an asymptomatic state. Three of the patients did not present symptoms of pancreatitis, however, as there were clinical data and elevation of said enzymes, a computerized axial tomography (CT) [13] was performed to confirm the diagnosis of pancreatitis [14]. (Table 1) shows the total and cumulative doses of L-Asp administered in patients at the time of diagnosis of pancreatitis, as well as the levels of pancreatic amylase and lipase enzymes. As part of the follow-up, the activity of L-Asp was measured in three different occasions, which were, after administration, during the absorption and elimination phase of the drug. After the quantification of the L-Asp activity in the three times, the data of the population PK/PD model previously validated in CECAN-Durango, Mexico were entered. Subsequently, three Asp concentrations were quantified at three different times, they were entered into the same PK/PD model previously validated in CECAN-Durango.
Table 1. Total and cumulative L-Asp doses of the patients at the time of diagnosis of pancreatitis, as well as amylase and lipase enzyme levels before and after diagnosis.
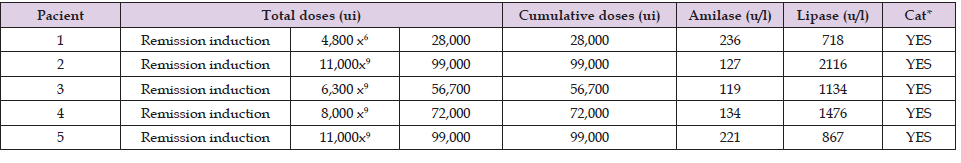
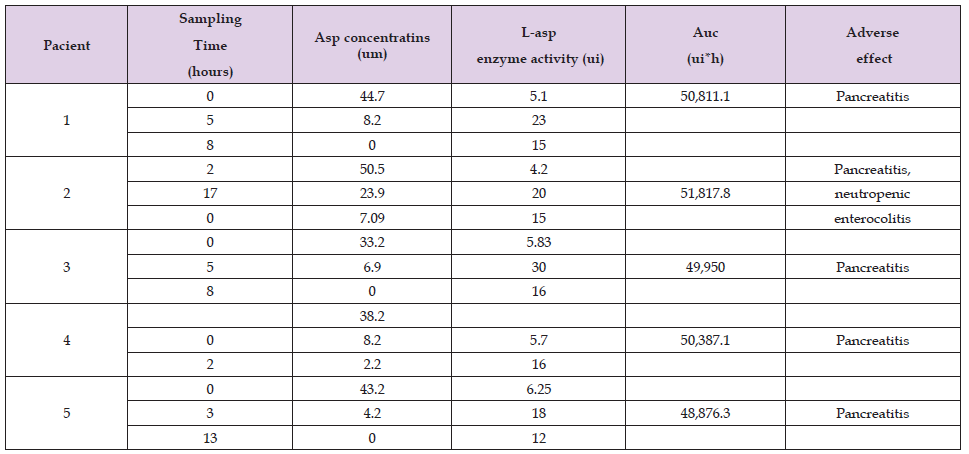
This follow-up was carried out, to determine the PK/PD parameters of L-Asp in the patients, and thus be able to adjust the dose of this drug, based on population PK/PD parameters and individual dose adjustment. (Table 2) shows the L-Asp activity, as well as the Asp levels found in the patients at different times. Patients one, three, four and five presented pancreatitis during the remission induction stage and patient number two, also presented neutropenic enterocolitis. (Figure 1) shows the Asp depletion profiles of the patients with pancreatitis, and at the same time, the profiles of the other patients who did not present pancreatitis but who were likewise followed up and monitored in the remission induction stage since December 2021 (30 patients). We observed that the patients who presented pancreatitis had an average slope lower that the patients who did not present pancreatitis, we did not find significant differences between the presence of pancreatitis and the area under the curve (AUC). (Figure 2) shows the enzymatic activity profiles of L-Asp in the treated patients. The graph shows that the patients who developed pancreatitis had an absorption phase in the first 5 hours, with a maximum peak between 2 and 5 hours after L-Asp administration. The rest of the patients had an absorption phase between the 10th and 18th hour after L-Asp administration, in both cases followed by an elimination phase that extends to 35 hours on average according to the simulations. Once pancreatitis was diagnosed in patients 1 and 3 treatment was suspended to reverse the adverse effects, and then continued, with intramuscular (IM), administration, with the monitoring of the patient’s pancreatic enzyme levels. For patients 4 and 5 the route of administration was changed to Intravenous (IV) and then continued with the monitoring of the patients’ pancreatic enzyme levels. Finally for patient 2, it was decided to completely suspend L-Asp administration.
Table 2. Both L-Asp activity and Asp levels found in patients at different sampling times are shown.
The understanding of developing pancreatitis from the use of L-Asp in children is limited and contradictory, adverse events have always been a major concern during the chemotherapy period. The aim of this case report was to describe our experience with five patients with ALL who developed L-Asp-induced pancreatitis during their remission induction stage. Studies on pediatric L-Aspassociated pancreatitis are limited, largely due to differences in pharmacokinetic characteristics and administration procedures [15]. There are currently three forms of L-Asp used in clinical practice: native and pegylated forms derived from Escherichia coli and an enzyme isolated from Erwiniachrysanthemi, known as Erwinia asparaginase [16]. Normally the administration of L-Asp reduces plasma Asp (amino acid) concentrations by catalyzing the deamination of Asp into aspartic acid [17]. Sufficient levels of L-Asp enzyme activity results in complete depletion of serum Asp concentrations, depriving leukemic blasts of this amino acid [18]. The lack of the amino acid Asp results in reduced protein synthesis and consequently leukemic cell death. Although it has played an important role in increasing the survival rate of childhood ALL, it can cause adverse effects including hypersensitivity reactions, pancreatitis, hyperglycemia, hypertriglyceridemia, and thrombosis [4,19,20]. The precise pathophysiology behind pancreatitis due to the use of L-Asp is unknown [21,22]. Some authors have mentioned that the reduction in protein synthesis resulting from L-Asp-induced Asp depletion could lead to toxicity, particularly in organs associated with high protein production, such as the liver and pancreas [3-23].
This may make sense, with our findings, in our work since of the 5 patients who developed pancreatitis all reached a depletion early in comparison with the patients who did not develop pancreatitis due to L-Asp. In this sense Pieters R [24] and collaborators mentioned in their study, that on average the patients to whom L-Asp is administered reach complete depletion after day 12 of the administration of L-Asp, this makes us suppose that such a fast depletion in the patients could represent a toxic effect in the pancreas. In relation to this, Sans MD and collaborators in 2021 [25] demonstrated in their study that amino acids are important regulators of digestive enzymes, and their deficiency could induce pancreatic insufficiency, especially Asp within the non-essential amino acids. On the other hand, in our work we found that patients who developed pancreatitis had a rapid L-Asp absorption phase of 5 hours while absorption in patients who did not develop pancreatitis ranged from 10 to 18 hours, this could have had an implication in the development of pancreatitis. It is important to point out that none of the patients who developed pancreatitis were positive for the presence of antibodies against L-Asp, for this reason we believe that the development of pancreatitis due to L-Asp may have a genetic influence, as mentioned by Grimes AC and collaborators in 2021 [26] in their study of genetic markers of risk for pancreatitis in Hispanic children with leukemia. These results lead us to continue monitoring patients administered with L-Asp at CECAN-Dgo, Mexico, in the search for validation as a marker of risk of developing pancreatitis due to L-Asp. From the results obtained, it seems that pancreatitis is related to the intensity of the L-Asp dose as can be seen in (Figure 1) and the decrease in Asp in (Figure 2), as if they were a mirror image.
The results show the PK/PD profiles of L-Asp and Asp respectively in five patients with ALL, under treatment with L-Asp. The pharmacodynamic profiles of patients who developed pancreatitis show a much shorter time in the rate of Asp depletion than the slope observed in patients who did not develop pancreatitis. Thus, the Asp depletion slope could be used as a risk marker for developing pancreatitis in conjunction with dose intensity in those patients administered L-Asp.
The authors declare that they have no conflict of interest due to
the publication of this article.
The authors thank the National Council of Humanities, Sciences
and Technologies (CONAHCYT) for financial support.
The authors thank the State Council for Science and Technology of
the State of Durango (COCYTED) for financial support.


