Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Karishma Shah, Shivprakash Rathnam, Harsh Rathnam and Kilambi Pundarikakshudu*
Received: May 18, 2023; Published: May 26, 2023
*Corresponding author: Kilambi Pundarikakshudu, Research and Development Department, Avance Phytotherapies Pvt. Ltd., 33-34, The Chamber Building, Opp. New Gurudwara, S.G. Highway, Ahmedabad-380 054, India
DOI: 10.26717/BJSTR.2023.50.007990
Context: Curcumin has been reported to be an effective and safe molecule for the treatment of a number of cancers. But its utility was restricted due to its poor water solubility and poor bio-availability due to degradation.
Aims: The objective of the present work was to test some of the water soluble conjugates of curcumin on Human colorectal adenocarcinoma and Human hepatocellular carcinoma cells
Settings and Design: Five Human colorectal adenocarcinoma cell lines and two Human hepatocellular carcinoma cell lines were treated with eleven different conjugates of curcumin.
Methods and Material: Human colorectal carcinoma cell lines of ATCC (SW 620, Colo 205, HT 29, HCT-15 and Caco-2) and Human hepatocellular carcinoma cells (SK-Hep-1 and Hep-G2) were seeded in to 96 well plates at a density of 5 x 103 cells per well. The cells were incubated for 72 hours with 0.001 μg/mL to 250 μg/mL of the test compounds. Cytotoxicity of the cells was determined in vitro by addition of MTT and incubation for 3 hours.
Statistical Analysis Used: Mean and SEM were calculated for cytotoxicity results.
Results: Of all the compounds tested, leucine and iso-leucine conjugates had better results over other compounds. Higher concentrations of 250 μg/mL of certain compounds exhibited cytotoxicity on Human hepatocellular carcinoma cells. Of all the cell lines HT 29 and Caco-2 cell lines exhibited highest cell death at very low concentrations of the test compounds. SK-hep-1 cells are more susceptible to the compounds than Hep-G2 cells.
Conclusion: Amino acid di-ester conjugates of curcumin are promising candidates for targeting different types of colon and liver cancers.
Keywords: Curcumin Amino Acid Conjugates; Colorectal Adenocarcinoma Cells; Hepatocellular Carcinoma Cells; Cytotoxicity; MTT Assay
Key Messages: Curcumin amino acid conjugates have shown promising cyto-toxicity on different Human colorectal adenocarcinoma and Human hepatocellular carcinoma cell lines at low concentrations in a MTT assay. This paves the way for exploring the usefulness of these compounds in the treatment of various cancers.
Curcumin (diferuloylmethane; 1,7-bis-(4-hydroxyl 3-methoxyphenyl)-1,6-heptadiene-3,5-dione) (Figure 1) promising anti-cancer activities against prostate, breast, pancreatic, head and neck, skin, liver, lung, colon and stomach cancers both in vitro and in vivo [1-5]. Curcumin was reported to act by diverse mechanisms such as inhibiting or down-regulating intracellular transcription factors like NF-кB, activator protein -1(AP-1), cyclooxygenase II (COX-II), nitric oxide synthase, matrix metalloproteinase-9 (MMP-9), STAT 3, TNF-α etc. [1,6] Curcumin was also shown to bring about cell cycle arrest in the G2/M phase in HCT 116 colorectal cancer cells [7]. It inhibits proliferation and promotes apoptosis through cleavage of bcl- 2 member protein, cytochrome c release and activation of caspases [8]. The presence of two phenyl rings with a 7-C linker having β-diketo function (C=O) groups as hydrogen acceptors and C-4 as hydrogen donor are considered to be essential in the binding of STAT 3 leading to apoptosis in H-Ras MCF 10 A cells [9,10].
Curcumin has low water solubility which impairs its chemical stability and oral bioavailability [1]. Kinetic stability of curcumin could be enhanced by glycosylation of the pharmacophore aromatic ring and also by masking the 4-OH groups [6,11]. Curcumin acetates and amino acid conjugates were shown to elicit anti-cancer activity by inhibition of proteasomes [12]. Chemical modification of curcumin involving methylene group or C=O did not show any advantage as far as anti-tumour activity is concerned. Hence, biodegradable ester linkage at phenolic hydroxyls is a promising proposition. Amino acid conjugates of curcumin have improved water solubility and better absorbed than curcumin. The conjugates also exhibited higher antibacterial, anti-fungal and antioxidant properties. Di-ester amino acid conjugates of curcumin exhibited higher activity than the monoesters [13,14]. Keeping in view the above facts, we undertook a preliminary in vitro cytotoxicity screening study of potassium salt of curcumin mono-sulphate, lipoic acid, leucine, phenylalanine, isoleucine, alanine, valine, proline, glucose, phenyl glycine, and glycine di-ester conjugates of curcumin on human colorectal adenocarcinoma and human hepatocellular carcinoma cells by employing MTT assay. The percent cytotoxicity and IC50 of these compounds on the cell lines are presented and discussed in this communication.
Chemicals, Media and Cell Lines
All chemicals and solvents used in the experiments were of analytical grade. The cancer cell lines for human colorectal adenocarcinoma (Colo205, HT-29, SW620, HCT-15, Caco-2) and Human hepatocellular carcinoma (Sk-Hep-1 and Hep-G2) of American Type Culture Collection (ATCC) origin were used in the experiments. Fetal Bovine Serum, Penicillin/Streptomycin are from Himedia, (Mumbai), Trypsin and EDTA are from Sigma Aldrich, U.S.A. Dulbecco’s Modified Eagle Media (DMEM) is from Caisson (U.S.A.); Eagle Minimal Essential Media (EMEM) is from Hi-media, Mumbai; Roswell Park Memorial Institute -1640 is from Hi-media, Mumbai; Leibovitz L 15 (Thermo Fisher Scientific, India) McCoy’s 5A Caisson (U.S.A.); Dimethyl Sulphoxide (DMSO) is from Merck, India; Doxorubicin is from RPG Life Science Ltd., (Mumbai). MTT (3-(4,5 dimethyl thiazol- 2yl)-2,5-diphenyl tetrazolium bromide is from Sigma-Aldrich, U.S.A. The codes and names of various curcumin conjugates are presented in (Table 1).
Cell Cultures and Media
The cell cultures are maintained in the media as described below.
Colo-205 (RPMI- 1640 +10% FBS), HT29 (McCoy’s 5A +10% FBS); SW 620 (Leibovitz L 15 + 10% FBS); HCT 15 (RPMI -1640 +10% FBS); Caco 2 (EMEM + 10% FBS); HepG2 (EMEM + 10% FBS); SK-Hep-1 (DMEM + 10% FBS). The cells were cultured in a carbon dioxide chamber with 5% CO2, 37oC temperature and 95% humidity.
Test Sample Preparation
The curcumin conjugates were accurately weighed and dissolved to get a stock of 20 mg/mL in DMSO and further diluted in serum free media to get concentrations of 250 μg/mL, 100 μg/mL, 10.0 μg/mL, 1.0 μg/mL, 0.1 μg/mL, 0.01 μg/mL and 0.001μg/mL.
Test Sample Administration
20 mL of each sample was added to cells in triplicate wells corresponding to the final concentration over a range of 0.001 μg/ mL – 250 μg/mL. Untreated cells served as control. DMSO served as vehicle control. The samples were administered once at the beginning of the experiment and incubated at 37oC for 72 hours.
Estimation of Cytotoxicity in Cell Lines
All the cell types were seeded at a density of 5 X 103 per well in 96 well plates (BD Falcon). After incubation of cells with the test item, the viability of the cells was determined by MTT based assay. 20 mL of 5 mg/mL of MTT in DMSO was added to all the wells and incubated at 37oC for three hours. The supernatant was aspirated and 150 mL of DMSO was added to all wells to dissolve formazan crystals. The optical density of each well was measured at 540 nm on a UV-visible Multi-well spectrophotometer (Mettler Toledo).
% Cytotoxicity was measured using the following formula:
% Cytotoxicity = (1-X/R)*100
Where X = OD of treated
R = OD of Controls (No test sample added).
The percent cytotoxicity of the various cancer cell lines on treatment with different curcumin conjugates is depicted in (Figure 2) (A-G). IC 50 values of the curcumin conjugates are compiled in (Table 2). Curcumin dilipoate
Table 2. IC 50 of curcumin analogues on Human colorectal adenocarcinoma and Hepato-cellular cancer cells.
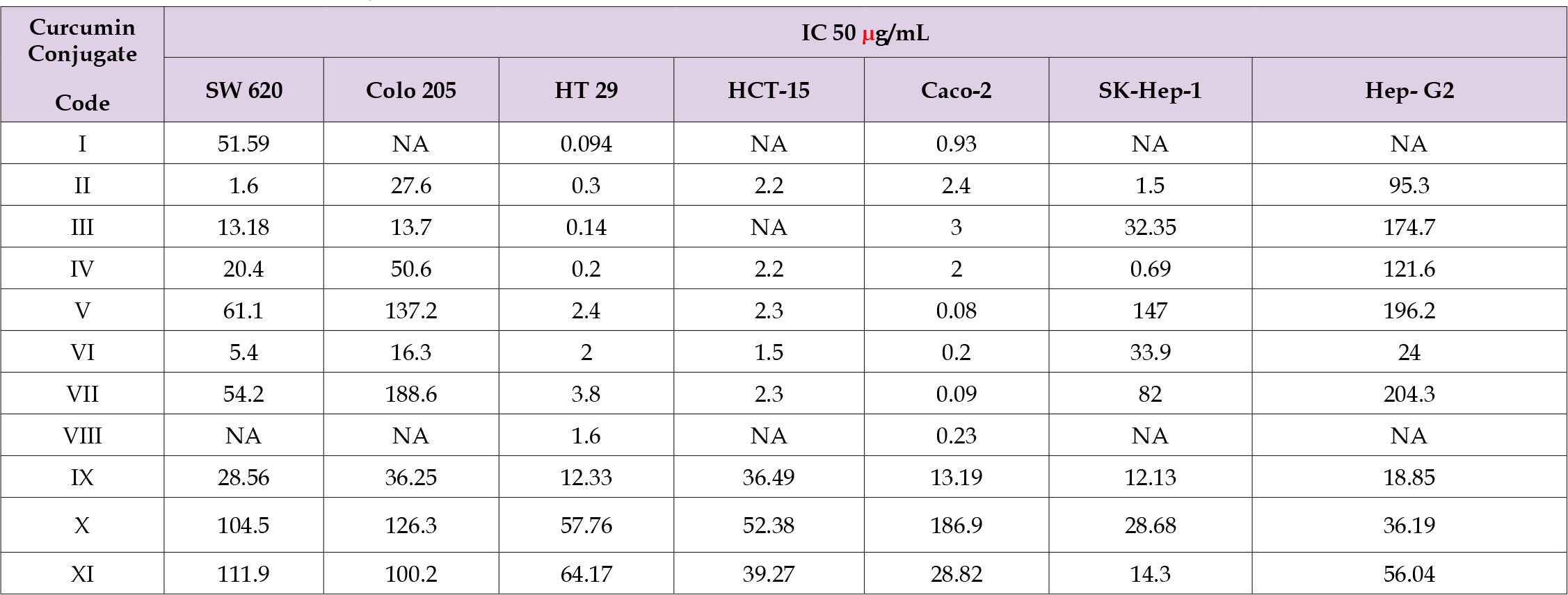
Note: NA: Not Available.
1. Exhibited highest cytotoxicity in HT-29 cells followed by
Caco-2 cells and SW 620 cells. A reasonably good activity of
this compound could be obtained even at a low concentration
of 0.01 μg/mL. IC50 of this compound was found to be 0.093
μg/mL and 0.094 μg/mL on Caco-2 and HT-29 respectively.
This compound did not show any activity on HCT-15, SKHep-
1 and HP-G2 cells. Leucine conjugate of curcumin
2. Showed potent cytotoxic activity on Colo 205, Caco-2, HT
-29, SW 620 cell lines of colorectal adenocarcinoma with
IC 50 of 27.6, 2.4 , 0.30 1.6 μg/mL respectively. A uniform
and substantial activity could be observed on SK-Hep-1
cells with highest cytotoxicity of 74.6% observed at a
concentration of 250 μg/mL and IC 50 was found to be 1.50
μg/mL. It showed very mild cytotoxicity on Hep-G2 cells at
lower concentrations while at 100 μg/mL and 250 μg/mL
concentrations it showed 44.7% and 79.4% cytotoxicity with
IC 50 of 95.3 μg/mL. There was no effect on HCT-15 cells.
Anti-proliferative effect on all the cell lines excepting HCT-15
was shown by curcumin phenylalanine conjugate
3. There was 50.7% cytotoxicity of HT-29 cells at a
concentration of 0.001 μg/mL and 250 μg/mL brought about
93.67% cytotoxicity. There was 74.4% and 79.6% cell death
of 100 μg/mL and 250 μg/mL concentration on SK-Hep-1
cells and 74.6% cytotoxicity at 250 μg/mL on Hep-G2 cells.
IC 50 of this compound was fond to be 0.14μg/mL and 3.0
μg/mL on HT-29 and Caco-2 cell respectively. More than 70%
cytotoxicity on all but HCT-15 cells lines was noted with 250
μg/mL of curcumin isoleucine conjugate
4. The activity being very striking on HT-29, SW 620, Sk-Hep-1
an Hep-G2 cell lines. IC 50 of this compound on HT- 29 and Sk-
Hep-1 cell lines was found to be 0.2 μg/mL and 0.69 μg/mL
respectively. At a low concentration 0.01 μg/mL, curcumin
alanine conjugate
5. Evinced 47.2 % and 43.3% cytotoxicity on HT-29 and Caco-
2 cells. Cytotoxicity on hepatocellular cancer cells SK-Hep-1
and Hep-G2 at 250 μg/mL was found to be 79% and 65.5%
respectively. HCT-15 cells proliferation was arrested by this
compound. Valine di-ester of curcumin
6. Was found to be very effective on Colo-205, SW 620, HT-29
and Caco-2 cells. At 0.01 μg/mL the di-ester showed 45.4%
30.0% cytotoxicity on Caco-2 and HT- 29 cells in that order.
At 100 μg/mL it was cytotoxic to 90 % of Colo-205 cells and
more than 70% of Sk-Hep-1 and Hep-G2 cells. IC 50 of this
ester on Caco-2, HCT-15 and HT-29 cells was found to be
0.08 μg/mL, 2.3 μg/mL and 2.4 μg/mL respectively. Proline
conjugate of curcumin
7. Was found to be highly cytotoxic (50%) on Caco-2 and HT-
29 cells even at a very low concentration of 0.001 μg/mL. At
250 μg/mL, it had 93.7% cytotoxic activity. It was active on
other cell lines only at 250 μg/mL concentration. Its IC 50 on
Coco-2 was 0.09 μg/mL and on Ht-29, the value was 38 μg/
mL. Curcumin monosulphate potassium salt
8. Has effect only on Caco-2 and HT-29 cells with slightly more
than 40% cytotoxicity at lower concentrations of 0.01 μg/
mL. The IC50 values of this salt on these cells was found to be
0.23 μg/mL and 1.6 μg/mL respectively. Glucoside derivative
of curcumin
9. Has more than 60 % and 84% activity on all cell lines tested
at concentrations of 100 μg/mL and 250 μg/mL respectively.
The IC 50 values for this compound were found to be 12.13
μg/mL (Sk-Hep-1), 13.19 (Caco-2) and 12.33 (HT-29).
Curcumin phenyl glycine
10. Has very weak activity. It was effective on Caco-2 and Sk-
Hep-1 cells with IC 50 values of 186.9 and 28.68 μg/mL
respectively. Curcumin glycine conjugate
11. Was also found to be effective on Sk-Hep-1 (IC50 28, μg/mL)
and Caco-2 cells (IC50 14.30 μg/mL), the effect being more
apparent at 100 μg/mL and 250μg/mL concentrations.
(Sheng Biao Wan, et al. [12]) found that hydrochloride salts of bisglycinoyl, bis-alaninoyl and bis-valinoyl conjugates of curcumin, due to their higher water solubility, inhibited the chymotrypsin-like activities of proteasomes in prostate cancer cell lines PC-3 and LNCaP. (Dubey, et al. [14]) noted that diglycinoyl, divalinoyl, and diglutamyol esters of Curcumin exhibited higher apoptotic activity on HeLa (Cervical cancer) and KB (Oral cancer) cell lines. The activity was higher than that found with the mono-esters. They opined that accumulation and better stability of these conjugates facilitate the activation of caspases leading to apoptosis. As suggested by these authors, the conjugates render the molecule more water soluble, better penetrable into the cells and protection from metabolic degradation. These conjugates probably act as prodrugs and ensure better accumulation inside the cells where the ester linkages could be easily hydrolysed to release curcumin inside the cells. Thus our results corroborate the findings of previous researchers and suggest that amino acid conjugates have high cytotoxicity on various colorectal adenocarcinoma cells. Of all the cell lines studied, HT-29, HCT-15 and Caco-2 cells were more susceptible to the leucinoyl, isoleucinoyl, alaninoyl, vaninoyl and prolinoyl conjugates. Leucinoyl and isoleucinoyl conjugates also exhibited promising activity on hepatocellular carcinoma cells (Sk- Hep-1). This study indicates that various water soluble alkyl amino acid conjugates may be potential candidates in the quest for finding safe and effective molecules to treat colon and liver cancers. The underlying mechanisms responsible for their cytotoxic activity needs to be studied in greater detail.


