Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Lim Shan Rou1, Nabila Perveen1, Naeem Hasan Khan1 and Arunachalam Muthuraman2*
Received: May 16, 2023; Published: May 23, 2023
*Corresponding author: Arunachalam Muthuraman, Department of Pharmaceutical Chemistry, Faculty of Pharmacy, AIMST University, 08100-Bedong, Kedah D.A., Malaysia
DOI: 10.26717/BJSTR.2023.50.007974
Rutin is a bioflavonoid and plant pigment. It is found in various fruits and vegetables. Chemically, it is known as rutoside, quercetin-3-O-rutinoside, sophorin, and vitamin P. It has antioxidant, anti-inflammatory, and immunomodulation-associated neuroprotective and anti- Alzheimer actions. The role of rutin against glyphosate-induced cognitive dysfunction in adult zebrafish has not been studied yet. The present study focused on the role of rutin on glyphosate- induced cognitive dysfunction in adult zebrafish. The glyphosate (GYP, 0.5 mg/L), rutin (50, 100, and 150 μg/L), and donepezil (DP, 1 μg/mL) for 30 minutes/day were exposed for 14 consecutive days. On the 14th day, the neurobehavioural changes were assessed by three horizontal compartment test, optokinetic motor response (OMR), startle response (SR), and T-maze tests. On the 15th day, animals were sacrificed and brain biomarker changes i.e., brain acetylcholinesterase (AChE) activity, thiobarbituric acid reactive substances (TBARS), and reduced glutathione (GSH) levels were estimated. The exposure to rutin ameliorated the GYP-induced cognitive dysfunctions and changes in tissue biomarkers. The results are similar to DP-exposed animals. Hence, rutin can be useful for the treatment of herbicide toxicity like GYP-associated neurocognitive disorders viz anti-oxidant, anti- lipid peroxidation, and modulatory action of cholinergic neurotransmitter activities.
Keywords: Cholinergic Neurotransmitter; Optokinetic Motor Response; Reduced Glutathione; Startle Response Test; Thiobarbituric Acid Reactive Substances; Three Horizontal Compartment Test; T-Maze Test
Cognitive dysfunction is mainly indicating attention deficits, verbal and non-verbal learning impairments, amnesia, dementia, alteration of visual & auditory processing, difficulty to solve problems, and changes in motor functions [1,2]. As per the Centers for Disease Control and Prevention (CDC) report, the prevalence rate of cognitive dysfunctions is 11.1% of the population, or 1 adult in every 9 adult persons. In above 65 years aged population is 11.7% and 10.8% of patients between 45-64 years of age [3]. Environmental pollutants i.e., pesticides are one of the risk factors for the development of cognitive impairment [4]. Current experimental evidence also revealed that the herbicide also causes cognitive dysfunction in rats via synaptic impairment and hippocampal neuron damage [5]. Glyphosate (GYP, herbicide) is one of the neurotoxic agents and causes neuronal damage [6]. Furthermore, it also alters the neurotransmitter levels leading to inducing neurobehavioural alterations [7]. The higher concentration of GYP in the nervous system shows monoaminergic neurotransmitter alteration i.e., serotonin (5-HT, 5-hydroxytryptophan), dopamine (DA), and norepinephrine (NE) levels which are responsible for anxiety, fears, defensive, visual-motor functions [7,8]. Furthermore, the level of acetylcholine (ACh) neurotransmitter levels are also modulated by GYP via modulation of acetylcholinesterase enzyme activity which leads to alters the learning & memory functions [9]. The exposure of GYP is reduce the zebrafish brain ACh neurotransmitter level via enhancement of AChE activity and oxidative stress [9]. Zebrafish animal species is one of the common experimental research for the assessment of neurobehaviour assessment [10].
Rutin is a bioflavonoid and plant pigment. It is found in various fruits and vegetables. It is also known as rutoside, quercetin-3-Orutinoside, sophorin, and vitamin P [11,12]. The citrus flavonoid of rutin glycoside is a low molecular weight polyphenolic compound. It reduces cellular oxidative stress and induces neuroprotection against free radicals and 3-nitro propionic acid (mitochondrial toxin) in rats [13]. Besides, it also produces tissue and cell protection against fungicide (i.e., thiram) toxicity [14] and organophosphate pesticide (i.e., trichlorfon) associated neuronal damage and neurobehavioural in catfish [15]. Furthermore, rutin also regulates the 3-nitro propionic acid- associated changes of cholinergic, aminoacidergic, and monoaminergic neurotransmission in mice [16]. Besides, rutin ameliorates scopolamine-induced cognitive impairments by regulating the free oxidative stress and cholinergic neurotransmitter signalling actions [17]. Similarly, it protects the doxorubicin-induced neuronal damage and improves memory functions in Wistar rats [18]. Moreover, it is also evidenced to improve the spatial memory actions against amyloid-β oligomer-associated neuroinflammation and Alzheimer’s disease (AD) in transgenic mice [19]. However, the role of rutin action against glyphosate-induced cognitive dysfunction has not been explored yet. Hence, the present study was designed to explore the ameliorative potential of rutin on glyphosate-induced cognitive dysfunction in adult zebrafish.
Animals
A total of 120 male adult zebrafish 5-6 months old which were purchased from pet shops were used for this proposed study. The sample size of 20 zebrafish per group was chosen following the minimal number of animals used in previous studies to obtain statistically meaningful results. All the fish was placed in 6 aquarium tanks at a temperature of not more than 29°C. The Zebrafish in all the tanks were given standard micro pallet feed and fixed oxygen pumps which were purchased from a pet shop. The water in the tanks was changed every two days. The study was approved by AIMST University Animal Ethics Committee (AUAEC/FOP/2023/03).
Drug Treatment Schedule
To study the effects of glyphosate (GYP, 0.5 mg/L for 30 minutes/ day) for 14 days on zebrafish [20]; donepezil (DP, 1 mg/L for 14 days) exposure to zebrafish [21]; and rutin (50, 100 and 150 μg/L for 30 minutes/day) for 14 days to zebrafish [22].
Experimental Protocol
Six experimental groups were employed in this study. Each group consists of 20 adult male zebrafish (6 months old).
Group 1: Normal control
Group 2: Glyphosate (GYP, 0.5 mg/L for 30 minutes/day) was exposed for 14 consecutive days.
Group 3: Rutin (50 μg/L for 30 minutes/day) was exposed for 14 consecutive days in GYP exposed group.
Group 4: Rutin (100 μg/L for 30 minutes/day) was exposed for 14 consecutive days in GYP exposed group.
Group 5: Rutin (150 μg/L for 30 minutes/day) was exposed for 14 consecutive days in GYP exposed group.
Group 6: DP (1 μg/mL for 30 minutes/day) was exposed for 14 consecutive days in GYP exposed group.
On the 14th day, all the animal neuro behavioural patterns were evaluated. On the 15th day, animals were sacrificed, and brain tissue was collected & used for biochemical analysis.
Assessment of Behavioral Tests
All the behavioural tests i.e., three horizontal compartment test, optokinetic motor response (OMR), startle response (SR) and T-maze tests were carried out in 3rd week. Thereafter animals were sacrificed for the collection of a tissue sample for biochemical estimations.
Three Horizontal Compartment Test
A three-horizontal compartment test is a method for the assessment of neurocognitive function as described by Dubey et al. [23] with minor modifications of Rishitha and Muthuraman [24]. The water level in the TLC chamber (26.5 L x 7.5 W x 23.5 H; cm) was held at 21 cm, and it was divided into three horizontal compartments (7 x 7 x 7; cm height) by drawing a line in the outer chamber. Graph paper was covered at the back of the chamber. Separate training (60 seconds) was provided to all fish the day before to swim in all compartments. The cognitive function was assessed the next day by measuring “time spent in the lower segment” (TSLS). When an animal is put in the test chamber, it usually prefers to swim in the upper segment within 15 seconds. It implies that the animal’s memory capacity is normal or enhanced. If an animal does not choose the upper segment and swims in the middle or lower segment, the animal has likely lost memory.
Assessment of Optokinetic Motor Response (OMR)
The OMR test is one of the common tests for the assessment of visual behavior in zebrafish. This device makes the swimming tendency toward the moving black and white stripes as a described method of Fleisch and Neuhauss [25]. In brief, the OMR unit system consists of two circular acrylic drums, the inner of which is fully transparent and the outer of which is non-transparent. Both of them are set in a circular pattern. The transparent center chamber (120 mm in width and 100 mm in height) contains the pole (30 mm in diameter), water, and fish in the center. The water depth was held at 60 mm. Another non-transparent outer chamber (200 mm width and 100 mm height) is made up of uniformly sized black and white vertical stripes (10 mm width and 80 mm height). From the top corner, strips were placed inside. The outer chambers revolved at a pace of 10 revolutions per minute (RPM). As described by Ninkovic and Bally- Cuif, zebrafish in this state can “see” the moving stripes and swim in synchronization with them [26]. Furthermore, a DC motor operated the outer drum’s moment in either clockwise or counterclockwise directions. At the water surface level, the light intensity of the room light was also illuminated at 300 lux. The first visual stimulus was shown to the zebrafish in the middle chamber on the first day of the experiment. The baseline exposure lasted 1 minute and did not have any rotation of the outer chamber (stationary condition). Fish was allowed to swim against visual stimulus by rotating the outer chamber clockwise and anticlockwise for one minute respectively on the day of visual activity testing. With a correlation of the visual threshold of rods and cones cell function in zebrafish, the swimming duration (in seconds) was recorded as OMR.
Assessment of Startle Response (SR)
Zebrafish is a highly sensitive animal species and it is readily responding to environmental stimuli. The SR of zebrafish was described method of Kirshenbaum et al. [27]and Burton et al. [28]. The zebrafish were exposed in a transparent square chamber (26.5 L x 7.5 W x 23.5 H) with a 4 cm water depth. Graph paper was covered at the back of the chamber. At the front of the SR apparatus, a video camera was used to monitor the animal’s movements. In this chamber, the animal was able to acclimate for 1 minute. Before starting the SR testing, the number of line crossings over 2 minutes was recorded to determine the usual swimming pattern. For the induction of startle (sudden changes in body position and/or acceleration of the swimming pattern), a 2 minutes exposure to low-intensity red LED light (300 lux) was introduced. The number of line crossings within 2 minutes was used to determine the swimming pattern. Zebrafish were held in the center of the SR apparatus for the period of the experiment. SR responses were recorded with light stimulation after completing 1 minute of baseline exposure. The SR test was conducted from 8 AM to 9 PM. The percentage startle response was calculated by using the formula:
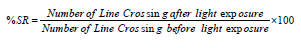
T-Maze Test
The t-maze test is another method for the assessment of neurocognitive function. The T-maze test is one of the conventional methods of memory assessment in rodent species. The t-maze test was performed as a described method by Buccafusco [29]. The T-maze test apparatus was modified by various researchers, and it produced reliable outcomes in zebrafish. Colwill, et al. [30] identified T- Maze tests for zebrafish, with slight modifications by Rishitha and Muthuraman [24]. The T-maze apparatus is made up of two small arms (10 Lx 6 W x 10 H cm) each with a different colour (one arm with red colour glass and the other end arm with green colour glass). One long arm (20 L x 10 W x 10 H; cm) is filled with the home chamber (5 L x 6 W x 10 H; cm) and is made of standard non- transparent glass. All animals were required to adapt to the study of the T maze environment and the animals were prompted to the middle of both short arms with a glass rod if they did not move from the corner of the long arm. Animals were led to the green chamber if they didn’t know how to reach the specific chamber. Animals were placed in the corner of the long arm the following day, which was the starting point from the home chamber, and the goal point was determined to be an entrance to either of the short arms. The learning and memory assessment, each fish was explored for two minutes. For the evaluation of cognitive function, the transfer latency (TL) was noted.
Estimation of Biomarkers Levels
On the 15th day, all the animals were sacrificed using tricaine mesylate (Tricaine methanesulfonate or MS-222) at a dose of 150 mg/L, and brain tissue was collected. The tissue homogenate was made with 1 ml of phosphate buffer saline (25 mM, pH 7.4). Then, the clear supernatant (aliquot) was used for biomarker estimation i.e., brain AChE activity, thiobarbituric acid reactive substances (TBARS), reduced glutathione (GSH), and total protein levels.
Estimation of AChE Activity
The AChE activity will be estimated by the spectrophotometric method as described by Ellman, et al. [31]. About 500 μl of the aliquot will be added in 0.25 ml (0.001 M) DTNB solution and incubated at room temperature (37°C) for 10 min. The variation of absorbance will be recorded from a spectrophotometer (DU 640B Spectrophotometer, Beckman Coulter Inc., California, USA). The 420 nm wavelength will be fixed for the assessment of absorbance changes. The total AChE activity levels will be calculated with the standard formula:
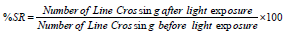
Here, AChE enzyme activity expressed ‘n’ mole of acetylthiocholine iodide hydrolyzed per minute in mg of protein; the volume of the assay is 3 ml; δ O. D. expressed the change of absorbance per minute; epsilon (ε) represents the extinction coefficient i.e., 13,600 per mol per centimeter.
Estimation of TBARS Level
The lipid peroxidation (LPO) product will be estimated by a spectrophotometric method as described by Okawa, et al. [32]. About, an aliquot (0.2 ml) will be added in 0.2 ml of sodium dodecyl sulfate (SDS, 8.1% w/v), 1.5. ml of acetic acid (30%; pH 3.5), 1.5 ml of thiobarbituric acid (0.8% w/v). The distilled water will be used for the maintaining of test tube volume (4 ml). The tubes will be incubated in a warm (95 °C) water bath for 1 h. Thereafter, test tubes will be cooled with tap water. Further, distilled water and 15% v/v of the n-butanol-pyridine mixture (1:5) will be added. After 10 min, tubes will be ultracentrifugation for 15 min with relative centrifugal force i.e., 1372 g of relative centrifugal force. The colour intensity of pink colour chromogen will be analyzed by spectrophotometer at 535 nm wavelength. The 0 nM to 10 nM of 1,1,3,3-tetramethoxypropane (TMP) was used as the reference standard.
Estimation of GSH Level
The GSH level will be estimated by a spectrophotometric method as described by Ellman [33]. About 0.5 ml aliquot was mixed with disodium hydrogen phosphate solution (0.3 M) and freshly prepared 0.001 Mole of 5,5’-dithiol-bis-(2-nitrobenzoic acid) (DTNB, Ellman reagent) solution. The color intensity of yellow chromogen will be noted by using a spectrophotometer at 412 nm wavelength. The 0 to 100 μM of reduced glutathione (GSH) was used as the reference standard.
Estimation of Total Protein Level
The total protein level will be estimated by a spectrophotometric method as described by Lowry et al. [34]. About 300 μl aliquot will be mixed with 700 μl of distilled water. Further, 5 ml of Lowry’s reagent will be mixed in test samples. The mixture will be incubated at 37°C for 15 min. Then, the Folin- Ciocalteu reagent solution (0.5 ml) will be mixed slowly and vortexed vigorously for 30 min. The colour intensity of purple chromogen will be noted by using a spectrophotometer at 750 nm wavelength. The 0 – 10 mg of Bovine serum albumin (BSA) was used as the reference standard.
Statistical Analysis
All the results were recorded as mean plus or minus standard deviation (± SD). Data secured from all behaviour tests and tissue biomarkers were statistically analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s multiple range test as posthoc analysis. The Graph pad Prism Version- 5.0 software was used for statistical analysis. A probability value of p < 0.05 was considered statistically significant.
Effect of Rutin in GYP-Induced Horizontal Compartment Response Changes
The exposure of GYP (0.5 mg/L for 30 minutes/day) for 14 consecutive days produced a significant (p < 0.05) impairment of neuro behaviour pattern in the horizontal compartment response test as an indication of increasing TSLS values when compared to the normal control group. The exposure of rutin (100 and 150 μg/L for 30 minutes/day) and donepezil (1 μg/mL) for 14 consecutive days were shown to have neuroprotective action against GYP-induced neuro behaviour changes. However, the treatment of rutin (50 μg/L) did not show significant attenuation of GYP-induced neuro behaviour changes. The results were illustrated in Figure 1.
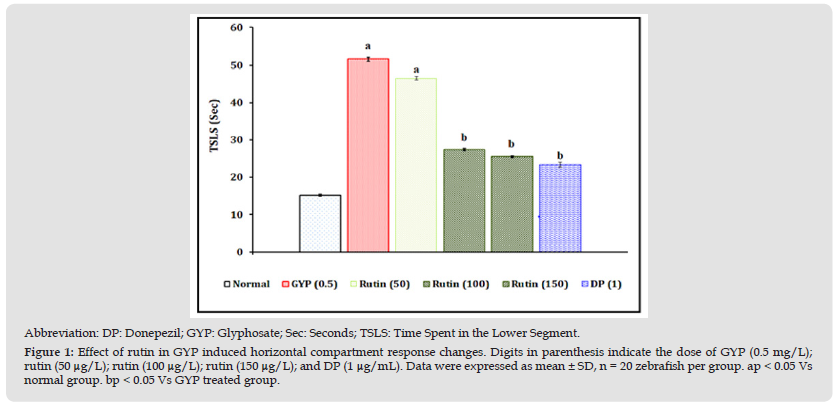
Figure 1 Abbreviation: DP: Donepezil; GYP: Glyphosate; Sec: Seconds; TSLS: Time Spent in the Lower Segment. Figure 1: Effect of rutin in GYP induced horizontal compartment response changes. Digits in parenthesis indicate the dose of GYP (0.5 mg/L); rutin (50 μg/L); rutin (100 μg/L); rutin (150 μg/L); and DP (1 μg/mL). Data were expressed as mean ± SD, n = 20 zebrafish per group. ap < 0.05 Vs normal group. bp < 0.05 Vs GYP treated group.
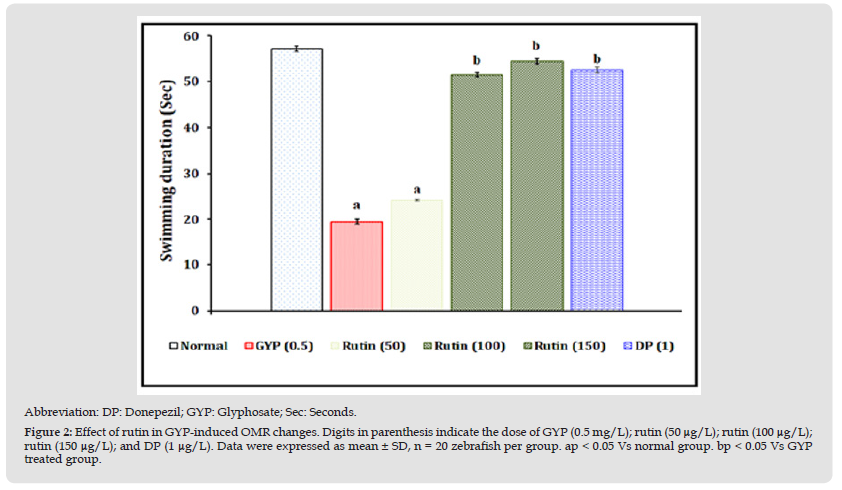
Effect of Rutin in GYP-Induced Optokinetic Motor Response (OMR) Changes
The exposure of GYP (0.5 mg/L for 30 minutes/day) for 14 consecutive days produced a significant (p < 0.05) impairment of visual-motor and neuromuscular functional patterns as an indication of decreasing swimming duration values when compared to the normal control group. The exposure of rutin (100 and 150 μg/L for 30 minutes/day) and donepezil (1 μg/mL) for 14 consecutive days were shown to have neuroprotective action against GYP-induced visual motor and neuromuscular functional changes. However, the treatment of rutin (50 μg/L) did not show significant attenuation of GYP-induced above the visual motor and neuromuscular functional pattern changes. The results were illustrated in Figure 2.
Figure 2 Abbreviation: DP: Donepezil; GYP: Glyphosate; Sec: Seconds. Figure 2: Effect of rutin in GYP-induced OMR changes. Digits in parenthesis indicate the dose of GYP (0.5 mg/L); rutin (50 μg/L); rutin (100 μg/L);rutin (150 μg/L); and DP (1 μg/L). Data were expressed as mean ± SD, n = 20 zebrafish per group. ap < 0.05 Vs normal group. bp < 0.05 Vs GYP treated group.
Effect of Rutin in GYP-Induced Startle Response (SR) Changes
The exposure of GYP (0.5 mg/L for 30 minutes/day) for 14 consecutive days produced a significant (p < 0.05) impairment of the defensive response pattern as an indication of decreasing percentage (%) SR values when compared to the normal control group. The exposure of rutin (100 and 150 μg/L for 30 minutes/day) and donepezil (1 μg/mL) for 14 consecutive days were shown to have neuroprotective action against GYP-induced % SR changes. However, the treatment of rutin (50 μg/L) did not show significant attenuation of GYP-induced above-defensive response changes. The results were illustrated in Figure 3.
Figure 3 Abbreviation: DP, donepezil; GYP, glyphosate; and SR, startle response. Figure 3: Effect of rutin in GYP-induced SR changes. Digits in parenthesis indicate the dose of GYP (0.5 mg/L); rutin (50 μg/L); rutin (100 μg/L);rutin (150 μg/L); and DP (1 μg/L). Data were expressed as mean ± SD, n = 20 zebrafish per group. ap < 0.05 Vs normal group. bp < 0.05 Vs GYP treated group.
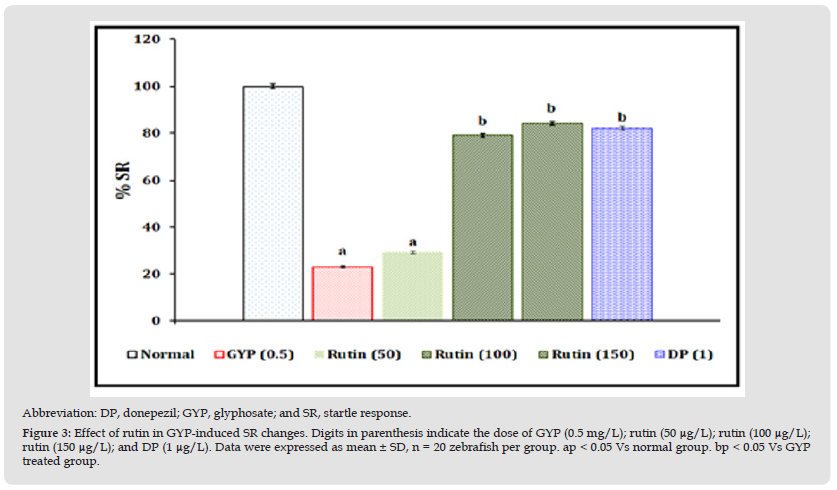
Figure 4 Abbreviation: DP: Donepezil; GYP: Glyphosate; Sec: Seconds; TL: Transfer Latency. Figure 4: Effect of rutin in GYP-induced T-maze test response changes. Digits in parenthesis indicate the dose of GYP (0.5 mg/L); rutin (50 μg/L);rutin (100 μg/L); rutin (150 μg/L); and DP (1 μg/L). Data were expressed as mean ± SD, n = 20 zebrafish per group. ap < 0.05 Vs normal group. Bp < 0.05 Vs GYP treated group.
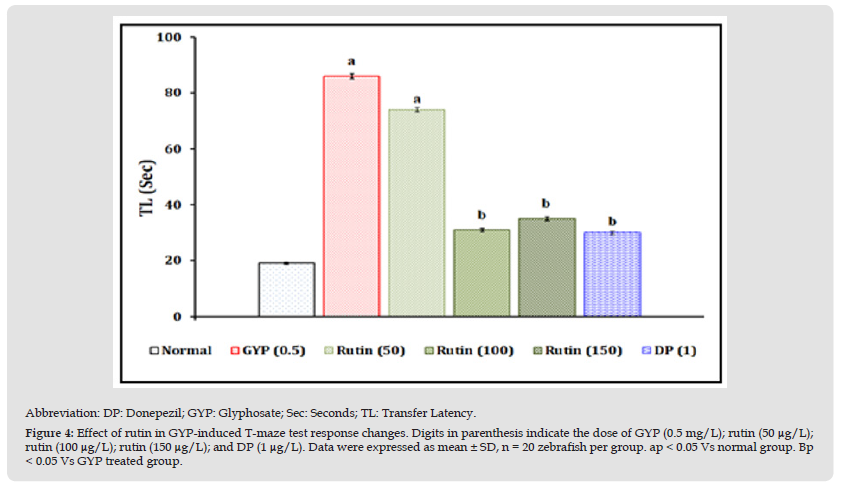
Effect of Rutin in GYP-Induced T-Maze Test Response Changes
The exposure of GYP (0.5 mg/L for 30 minutes/day) for 14 consecutive days produced a significant (p < 0.05) impairment of neurocognitive behaviour pattern as an indication of increasing TL values when compared to the normal control group. The exposure of rutin (100 and 150 μg/L for 30 minutes/day) and donepezil (1 μg/mL) for 14 consecutive days were shown to have neuroprotective action against GYP-induced TL values changes. However, the treatment of rutin (50 μg/L) did not show significant attenuation of GYP-induced neurocognitive behaviour changes. The results were illustrated in Figure 4.
Effect of Rutin in GYP-Induced Tissue Biomarker Changes
The exposure of GYP (0.5 mg/L for 30 minutes/day) for 14 consecutive days produced a significant (p < 0.05) raise in the brain AChE activity and TBARS levels and reduce the GSH levels when compared to the normal control group. The exposure of rutin (100 and 150 μg/L for 30 minutes/day) and donepezil (1 μg/mL) for 14 consecutive days were shown to the amelioration above tissue biomarkers changes. However, the treatment of rutin (50 μg/L) did not show significant attenuation of GYP-induced above biomarker changes. The results were expressed in Table 1.
Note: Digits in parenthesis indicate the dose of GYP (0.5 mg/L); rutin (50 μg/L); rutin (100 μg/L); rutin (150 μg/L); and DP (1 μg/L). Data were expressed as mean ± SD, n = 20 zebrafish per group. ap < 0.05 Vs normal group. bp < 0.05 Vs GYP treated group.
Abbreviation: AChE: Acetylcholinesterase Activity; DP: Donepezil; GSH: Reduced Glutathione; GYP: Glyphosate; TBARS: Thiobarbituric Acid Reactive Substances
The present study revealed the ameliorating effect of rutin against the GYP-induced neurobehaviour impairment in zebrafish like
1. Increased TSLS values in three horizontal compartment tests.
2. Decreased swimming duration in optokinetic motor response test.
3. A decreased percentage of SR values in startle response test, and
4. Increased TL (sec) values in T- Maze test. Besides, the levels of the biomarkers are also attenuated against the GYP toxicity i.e., increased brain AChE activity and TBARS levels; and decrease the GSH level. These results of rutin also mimic the reference drug i.e., donepezil actions. GYP is a known neurotoxic agent and cognitive impairments along with elevation of AChE activity [6,35]. The overactivation of AChE activity is hydrolyzing the acetylcholine to acetyl-CoA and choline molecules. The reduction of neuronal acetylcholine in cholinergic neurons and hippocampal area is causing cognitive impairments [7,36]. Furthermore, chronic exposure to GYP also alters the other neurotransmitter actions i.e., 5-hydroxytryptophan (5-HT), dopamine (DA), and norepinephrine (NE) levels which can cause a variety of neuronal dysfunctions and cognitive disorders [8,37]. Similar results were observed in GYP-induced cognitive impairments and activation of AChE functions in this research work.
Further, the exposure of GYP is enhance the oxidative stress associated neuronal damage via enhancement of acetylcholinesterase activity [9]. In addition, exposure to neurotoxicant i.e., chlorpyrifos cause oxidative stress and makes a great impact on acetylcholinesterase transcription in zebrafish embryos development and memory function in adult zebrafish [38,39]. Hence, pesticides and herbicides potentially alter the AChE activity and cellular radical homeostasis leading to cause neuroinflammation and memory dysfunction [40,41]. The current study also reveals that rutin attenuates the GYP-induced GSH (endogenous antioxidant agent) and oxygen radical-associated lipid peroxidation. GSH and TBARS are major hallmarks of cellular oxidative stress and defensive action against the reactive oxygen species (ROS) [42]. The ROS is playing a vital role in the regulation of neuronal functions [43]. At the lowest concentration, radicals are accelerating the neurotransmitter function and transmission of nerve impulses via neuroimmune cell networks [44,45]. However, the largest concentration of ROS known to cause neuronal damage via the activation of multiple neuronal prion proteins and unregulated neuronal apoptosis process & ferroptosis actions [46,47]. Rutin is a natural antioxidant molecule known to reduce ROS and neuronal damage [48]. Experimental evidence also revealed that rutin inhibits free radicals generation and neuroinflammation which leads to regulates the multiple cellular signaling process. Rutin reduces the oxidative stress markers in neuronal tissue against the 3-nitro propionic acid [13], thiram [14], trichlorfon [15], and trimethylene glycol dimethacrylate toxicities [49]. Similar results were also revealed in this study, rutin reduced lipid peroxidation and enhanced the GSH levels. Moreover, some of the limitations like evaluation of the additional molecular pathways with relevant expression protein analysis, toxicity potential assessment via histopathological analysis, and neuroprotective action of rutin can correlate with analysis of brain areas and neurotransmitters-based assessment. The extension of current research work is under investigation to address the above limitation-related issue.
Rutin possesses the potential ameliorative actions against the GYP-induced cognitive dysfunction viz reduction of free radical generations, lipid peroxidation, and regulation acetylcholine neurotransmitters. Hence, it may be a useful nootropic agent for herbicide-associated neurotoxicity and cognitive disorders.


