Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Mohamed Shafi Bin Mahboob Ali*
Received: May 10, 2023; Published: May 19, 2023
*Corresponding author: Mohamed Shafi Bin Mahboob Ali, Department of General Surgery, Hospital Universiti Sains Malaysia
DOI: 10.26717/BJSTR.2023.50.007964
Colorectal cancer (CRC) is a type of cancer in the human large intestine. Colorectal cancer is the third and second most common cancer, respectively, in men and women worldwide [1]. The number of colorectal cancer cases is expected to increase by 80% by 2035, climbing to approximately 2.4 million new colorectal cancer cases and contributing to 1.3 million deaths worldwide [2]. Colorectal cancer (CRC) is the commonest cancer in Malaysian men (age-standardized incidence rate 14.8/ 100,000), the second most common cancer in Malaysian women (age-standardized incidence rate 11.1/ 100,000) [3]. Unfortunately, in Malaysia CRC is detected late due to several factors. According to the research conducted in Malaysia, factors that cause delay in diagnosing CRC are lack of awareness, denial, negative perceptions of the disease, over-reliance on traditional medicine, misperceived risk, emotional barriers and negative perceptions towards screening [4-6]. Mortality due to colorectal cancer is increasing, and it is the fourth leading cause of cancer death in the world [1]. The ‘rise’ of colorectal cancer in developed countries can be attributed to the increasingly ageing population, unfavorable modern dietary habits and an increase in risk factors such as smoking, low physical exercise and obesity. The development of CRC has been linked with C-reactive protein and Interleukin-6, which are systemic marker of inflammation. Several prospective studies have investigated the association of prediagnostic circulating C-reactive protein concentrations with the development of colorectal cancer, but the results have been inconsistent [7].
Many research have been concentrated in studying the role of Interleukin-6,which is a type of hematopoieticmediated inflammatory cytokine that activates lymphocytes in the initiation of colorectal cancer [8]. Other factors that may promote the development of colorectal cancer such as growth factors, cytokines, and genetic or epigenetic alterations in specific oncogenes or tumor-suppressor genes, play a role during the adenoma-carcinoma sequence [9].
Abbreviations: IBD: Inflammatory Bowel Disease; HUSM: Hospital University Sains Malaysia; SOPD: Surgery Out-Patient Department; ELISA: Enzyme Linked Immunosorbent Assay; SD: Standard Deviation.
Research Design
Cross-sectional study.
Study Area
Department of General Surgery, Hospital University Sains Malaysia.
Protocol JEPeM Code
USM/JEPeM/21070514.
Study Population
We recruited a sample of 46 patients that were diagnosed with colorectal cancer by the means of colonoscopy examination, imaging as well as clinical. The presence of colorectal cancer were confirmed with histopathological examination of with a rectal biopsy or colonoscopy prior to biopsy. All these patients required surgical interventions for their diseases. Out of 46 patients, 28 patients were male while the remaining 18 patients were females.
Subject Criteria
Inclusion Criteria:
1. Age between 18-80 years old
2. Newly diagnosed or established diagnosis of colorectal cancer- by colonoscopic examination/imaging/histopathological
3. Any stages of colorectal cancer (stage 0-4)
Exclusion Criteria:
1. Mentally not sound to give consent
2. Inflammatory bowel disease (IBD)
3. Damaged/defective blood samples
4. Presence of another malignancy in the same patient-synchronous tumor
Withdrawal Criteria
• Change of Mind
• Found to be outside the parameters of inclusion criteria and inside the parameter of the exclusion criteria
• Breach of Protocol
Sample Size Estimation
Sample size were calculated for each objectives with type 1 error of 5%, Type 2 error of 20 %(80% power of study) and ratio of control to the experimental group. For objective 1, no sample size calculation needed as descriptive analysis will be used to describe the sample only. For objective 2, no sample size calculation needed as descriptive analysis will be used to describe the sample based on the grouping only, no hypothesis testing needed. For objective 3, The sample size calculation was done using “Sample size calculator 2.0” by Arifiin (2017) for sample size of specific objective 3 (Pearson’s Correlation-Hypothesis Testing) based on parameter’s value from Hidayat, et al. [10]. The calculation was done using all the parameters listed below:
Sample size calculation: Using Pearson’s Correlation-Hypothesis Testing
Expected correlation(r): 0.4
Significance level (α):0.05
Power (1-β):80%
Sample size=46
Expected dropout rate: 10%
Sample size with additional 10% dropout: 52
Reference Literature
Hidayat F, Labeda I, Sampetoding S, Pattelongi IJ, Lusikooy RE, Warsinggih, Dani MI, Mappincara, Kusuma MI, Uwuratuw JA, Syarifuddin E, Faruk M. Correlation of interleukin-6 and C-reactive protein levels in plasma with the stage and differentiation of colorectal cancer: A cross-sectional study in East Indonesia. Ann Med Surg (Lond). 2021 Jan 23; 62:334-340. doi: 10.1016/j.amsu.2021.01.013. PMID: 33552492; PMCID: PMC7847822. For objective 4, The sample size calculation was done using “Sample size calculator 2.0” by Arifiin (2017) for sample size of specific objective 4 (two mean formula) based on parameter’s value from Hidayat, et al. [10]. The largest sample size was taken as the final sample size and after anticipating 10% dropout rate, the corrected sample size was 46 patients.
Subject Recruitment
Subject recruitment-All patients that were previously diagnosed and was newly diagnosed with colorectal cancer by the means of colonoscopy, imaging and clinical, who fulfills the inclusion and the exclusion criteria’s by the Department of General Surgery in Hospital University Sains Malaysia (HUSM).
Subjects for the study was recruited from the following pool:
1. HUSM endoscopic suite
2. HUSM emergency department
3. Surgery out-patient department (SOPD)
4. Referrals from the nearby district hospitals in the Kelantan state
Randomization and Masking
No randomization or masking were applied in this study as this is a cross sectional study involving 46 patients who were confirmed to have colorectal cancer.
Research Tools
ELISA IL-6 Kit (Legend Max Human):
• Manufactured by Biolegend Way San Diego
• Expiry date is within 2 years of purchase
• Sensitivity: 0.8pg/ml
• Range: 1.56-50pg/ml
• Catalogue No: 430507
CRP Kit (QUIK READ GO CRP):
• Manufactured by Aidian Oy Koivu-Mankkaan tie 6 B, FI- 02200 Espoo, Finland
• Reproducible CRP results within a range of 5 - 200 mg/l
• Results within 2 minutes
• Lyophilized reagents containing <1% of sodium azide
• Catalogue No: 153763
Hettich Universal 32R Centrifugal Machine:
• The centrifuge machine that was used for the study
• Subject’s blood sample were centrifuge in the machine at 4500RPM for 5minutes to separate the serum from the red blood cells
PHCbi | MDF-DC700VX TwinGuard ULT Bench Top Freezer: The spun serum was kept in the freezer under temperatures between -2 degree to -28 degree Celsius.
CRP Analyzer Machine-Genes & Life Science Equipment’s (Model Number: MSLGH04):
• Analyze and interpret patient’s serum C-reactive protein levels at the speed of 60T/Hour
• Detection using laser device and integrated detection system
TC-96+ TECO Diagnostics Elisa Microplate Reader:
• The instrument carries out detections after it is conjugated with ELISA reagent
• First, it detects absorbencies of under detection sample and standard liquid
• Secondly, it performs analyses and calculations
• Finally, it produces values or conditions of the under detection material in the sample liquid
• The ELISA technique used was capture assay ‘sandwich’ method
• Optical density is used to detect the level of the primary antibodies bound to the proteins to at the bottom of the wells
• ELISA results can be displayed into three types: quantitative, qualitative and semi-quantitative
Data Collection Method
This cross-sectional study, conducted at the Hospital University Sains Malaysia (HUSM), followed a protocol approved by the JEPeM USM, which is an ethical body of the university with the registration code: USM/JEPeM/21070514, dated 20th December 2021 in accordance with the criteria of strengthening the reporting of cohort studies in surgeries [11]. A total of 46 patients who were diagnosed with colorectal cancer were recruited into the studies (28 males and 18 females) aged 56.11 years on average with mean value of 15.51. All these subjects needed surgical treatment due to CRC, the presence of which was confirmed by histopathological examination with a rectal biopsy or colonoscopy prior to biopsy. To be eligible to participate, patients had to have a diagnosis of CRC based on a histopathological examination, demonstrate normal liver function, kidney function, urinalysis and chest x-rays, have no history of hemostatic disorders, not currently suffering from acute or chronic infectious diseases as evidenced by routine blood results and consent to participate. Exclusion criteria were damaged blood samples or malignancy in another organs (i.e., synchronous tumor). Stages of CRC were determined with reference to the American Joint Committee on cancer’s 2017 TNM staging system [12], whereas grades of differentiation were determined based on the grading of the World Health Organization [13,14].
Sample Examination
Before the subjects underwent colorectal surgeries, they were counselled by the principal investigator regarding the details of the studies. Once the subject understood about the study details, consent will be taken from them. After obtaining written consent, about 3ml to 5ml venous blood will be withdrawn from the subject. Immediately the blood will be transferred to a yellow plain tube, label with subject’s unique ID and put in a biohazard bag. The principal investigator will then run the sample in a centrifuge machine for about 4500rpm for 5minutes. The obtained serum will be separated into two aliquots. One aliquot was stored at 080 degree for the later analysis of IL-6 serum concentration, namely with an enzyme linked immunosorbent assay (ELISA) kit (Legend Max Human) while another aliquot was tested for C-reactive protein serum level using CRP kit (Quick Read Go CRP).Once 46 samples obtained, the principal investigator run the test for both IL-6 and CRP on the same day with the help of HUSM lab assistant. IL-6 level were processed using sandwich ELISA method with the Legend Max Human kit according to the manufacturer’s instructions [15-17]. C-reactive protein was processed using Quick Read Go kit according to the manufacturer’s instructions. Measurement of IL-6 concentration was expressed in pictograms per milliliter (pg/ml) while CRP concentration was expressed in milligrams per liter (mg/L).
Data was entered and analyzed using IBM SPSS (Statistical Package for the Social Sciences) version 26. Data were screened for missing values prior to the analysis. From the total of 50 samples, a total of 46 useable sets of observations that were used for subsequent analyses. Then, descriptive statistics to describe the samples used in the present study was used. All categorical variables were summarized as frequency (n) and percentage (%), while numerical variables were presented as mean and standard deviation (SD). Then, subsequent analysis of descriptive statistic was used to demonstrate the difference mean Serum IL-6 between different stages of TnM. Then, Pearson correlation test was used to examine the relationship between mean levels of Serum IL-6 with mean CRP. The analysis was continued with summarizing the upregulation of Serum IL-6 in CRC in HUSM population between different aspects (stages, types of CRC and level of differentiation) using Kruskal-Wallis test with post-hoc comparison of Mann-Whitney test. It was to assess the difference in median levels of Serum IL-6 CRP at each stages, types and Differentiation level of CRC. The same analysis was used for examining the association between stages, types of CRC and differentiation level towards CRP level. The graphical illustration of bar chart, pie chart and box- plot were used to explain the distribution of all related variables individually or by combination for all the samples used in the present study. The scatter plot was used to show the relationship between mean serum level of CRP and Serum IL-6. All values less than 0.05 will be considered statistically significant.
Participants Characteristic
A total of 46 samples were included for this study. The data obtained was expressed as mean with standard deviation (SD) for numerical variables and frequency (n) with percentage (%) for categorical variables as tabulated in (Table 1).
Comparison of Serum IL-6 by Stages
Based on (Table 2), the mean serum IL-6 were different between different stages of the cancer. In general, the highest mean of serum IL-6 was found in stage I and the mean reduced with increase in level of stages.
Correlation of IL-6 and CRP
There was a significant positive moderate correlation found between IL-6 and mean value of CRP. Comparison of mean value CRP and serum IL-6 between different groups of variables. (Tables 3 & 4) shows the comparison of CRP and serum Il-6 between different stages, types of CRC and level of differentiation. Based on (Table 3), there were no significant association between related variables towards CRP value except for level of differentiation (p=0.026). The highest median value of CRP was found in well differentiation group (median= 96.00, IQR=89.00). Based on (Table 4), there were no significant association between related variables towards serum IL-6 value for stages, types of CRC and level of differentiation (all p>0.050).
Note: Kruskal Wallis test was applied. No IQR value for some variables because of only 1 cases or constant found in that group.
Note: Kruskal Wallis test was applied. No IQR value for some variables because of only 1 cases or constant found in that group.
Graphical Illustration of all Related Variables
All of the charts below illustrated and summarized the distributions of all related variables by each variable individually and in combination (Table 5).
We recruited 46 patients that were diagnosed with colorectal cancer. Out of 46 patients, 28 or 60.87% were males while 18 or 39.13% were females. We faced with recruitment limitations as the study was conducted during the COVID-19 pandemic era where most of the hospital resources and departments were concentrated in treating and managing COVID-19 patients. Thus, the level of screening and detections of colorectal cancer were drastically fell. The mean age of all 46 subjects were observed as 56. 11 years old with standard deviation of 15.51. This value is higher compared to the studies done by Hidayat, et al. [10]. Where the mean age was 50 years old. Male sex manifested the most for the development of colorectal cancer while ethnic wise, we observed that ethnic Malays has the highest numbers, followed by Chinese while no Indian ethnic was diagnosed with colorectal cancer in our centre. This findings corroborates with K-L Goh’s [17]. Data which gave us insight into Malaysia’s colorectal demographic trends. This trend is most probably due to the preponderance of the cancer to the elderly male while ethnic wise, Malay’s ethnic has the highest number due to the population factor that is the study was conducted in Kelantan, which is the East-Coast region of Malaysia with Malays majority. While different data was observed in Penang, a small island at the Northern region of Malaysia’s peninsular where the Chinese ethnic recorded the most number of cases in colorectal cancer [18]. We also observed high percentages in those colorectal cancer patients that are smokers and non-vegan.
This findings correlated with the findings of Nuri Faruk [19]. That processed red meat significantly increases the development of colorectal cancer about 20-30%. The mean CEA recorded in our subject was 214.04ng/ml with the standard deviation of 860.50, while the commonest region of tumor location was in colon compared to the rectum. The distribution of cancer stages were according the the cancer stages where stage 4 recorded the most, followed by stage 3,2 and lastly stage 1. Interleukin-6, which is a pro-inflammatory cytokine in the development of colorectal cancer, was studied their levels in different stages of colorectal cancers [20]. It was found that colorectal cancer stage 2 recorded the highest level of IL-6, Which was 326.67pg/ml with the standard deviation of 219.69,followed by colorectal cancer stage 1 with the value of 259.93pg/ml(S.D 370.66), Stage 4 with the value of 109.90pg/ml(S.D 160.95) and lastly, stage 3 with the Interleukin level of 79.49pg/ml(157.89). These findings were contraindicated with the study conducted by Heike Knüpfer which stated that higher IL-6 levels are associated with increasing tumor stages [21]. Subjects used in Heike Knüpfer was far smaller compared to ours and that may influenced the outcome. We found a significant positive moderate correlation between IL-6 and mean value of CRP with a Pearson correlation value of 0.603 and p value of <0.001. This proves that both IL-6 and CRP have significant roles in the development and progression of colorectal cancer. This findings is similar with the Greek studies on their colorectal cancer patients by Nikolaos, et al. [22].
We also studied the relationship of CRP and IL-6 with different variables such as the cancer stages, types and differentiation. We found that there were no significant association between CRP and the related variables except for the level of differentiation with the p-value of 0.026. On the other hands, there were no significant association between IL-6 and all related variables that were studied in the colorectal cancer. Both CRP and IL-6 correlation relationship were calculated using Kruskal-Wallis test. We tested both CRP and IL-6 with sandwich ELISA test and derived a scatter plot graph. The findings of the graph was a non-linear curve (Figure 1). CRP and IL-6 data for all 46 subjects were randomly scattered both on the upper and lower area of the plot. This shows that CRP and IL-6 have a non-linear relationship in the development of colorectal cancer. Our findings was totally different compared to the findings by our Indonesian colleagues F Hidayat, et al. [10]. In which they obtained a linear relationship between CRP and IL-6 in colorectal cancer patients. This can be due to many factors, such as the kit used, the lab technician that performed the test as well as the reagent used. Retrospective review of our data might shows a different result. (Figure 2) shows the graphical distribution of the colorectal cancer stages in which stage 4 has the most numbers, 30 subjects (65.22%), followed by the stage 3, 2 and lastly stage 1. While (Figure 3) shows us the different types of cancer found in our subjects which mostly were adenocarcinoma, 38 subjects (82.61%), followed by other types of cancer such as signet cell cancer.
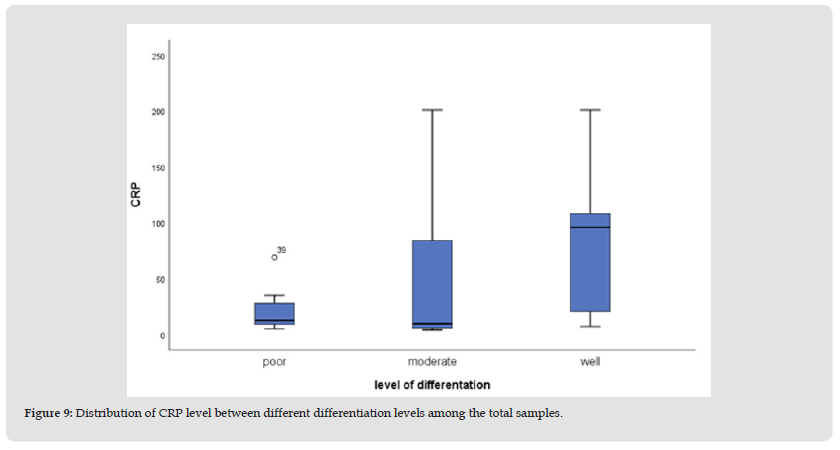
We had 2 subjects with hamartoma while 1 subject each for lymphoma and GIST. These findings were consistent with the cases that were commonly found in our centre which is Adenocarcinoma of the colon. Although other types of colon cancers have been reported, Adenocarcinoma takes the precedence. (Figure 4) shows the distribution of the types of differentiation of the cancer in which moderately differentiated colorectal cancer was the highest with 22 subjects (47.83%) followed by well differentiated and lastly was the poorly differentiated. This distribution indicates that most of our patients have a good to moderate prognosis. (Figure 5) depicting the levels of IL-6 amongst different stages of colorectal cancer in our study. We found that stage 4 has the highest IL-6 levels followed by stage 3, 2 and lastly stage 1. These findings corroborated with the studies done by Farideh Esfandi, et al. in which they concluded that the levels of IL-6 will be higher with the advancement of the cancer stages [23]. The box plot in (Figure 6) shows that plasma CRP in various colorectal cancer differentiation were not normally distributed and the mean level was highest in the well differentiated group. While box plot in (Figure 7) also shows that the level of CRP and the type of cancers were not normally distributed with other types of cancer documented the highest mean value. Box plot in (Figure 8) shows that CRP levels in different stages of cancer were not normally distributed either with stage 2 recorded that highest mean.
Figure 7 Distribution of Serum IL-6 level between different differentiation levels among the total samples.
Thus, there is no significant correlations between the CRP value and the CRC stages, types or differentiation. Similar findings were detected in (Figures 8-10) box plot for the IL-6 where there were no significant correlations between the IL-6 values and the stages, differentiation and the types of the CRC (Figures 11-15). These findings were consistent with a meta-analysis done by Bo Zhou, et al. Where they found that there were no direct correlation between CRP and IL-6 with the CRC development [24].
Figure 9 Distribution of CRP level between different differentiation levels among the total samples.
The study is confined to a single center with a limitation of the varieties of subject. Majority of our subjects were Malays as the study was conducted in HUSM Kubang Kerian, Kelantan where the majority population were Malays. Other races such as Chinese and Indians were very scarce making the studies racial distribution uneven. Our sample size were also small, only 46 subjects as the studies was conducted during the COVID-19 pandemic. Many patients were turned back as the infection rates and the number of cases surges. Other limiting factor was the duration of the studies was quite short. Better outcome might be produced if the study could be extended. Some of the samples were unevenly distributed due to small number of subjects and other factors.
Our study demonstrated that there is a significant positive moderate correlation between IL-6 and mean value of CRP with Pearson correlation value of 0.603 and a p-value of <0.001. Otherwise, there were no significant correlation between CRP value and the CRC related variables except for the cancer differentiation. There were no correlation at all between IL-6 and all CRC related variables. The relationship between IL-6 and CRP in the development of colorectal cancer may be used to diagnose early-stage CRC and thus, improve its management and the life expectancy of the patients.


