Impact Factor : 0.548
- NLM ID: 101723284
- OCoLC: 999826537
- LCCN: 2017202541
Osah Martins Onwuka1*, Erememi Noble Bonnie1, Nkechi Clara Nwosu2, Adaobi Linda Okerulu1 and Augustine Amadikwa Uwakwe3
Received: May 08, 2023; Published: May 18, 2023
*Corresponding author: Osah Martins Onwuka, Human Physiology Department, College of Medicine and Health Sciences, Gregory University, Uturu, Abia State, Nigeria
DOI: 10.26717/BJSTR.2023.50.007963
Background: Appetite plays role in determining food intake and its suppression could reduce the calories of food consumed, which can impact on body weight. Camellia sinensis and its products such as green tea, black tea as well as Lipton tea were reported to be potent in weight modulation, but its mode of action is not fully elucidated. Hence, the impact of Lipton tea on ghrelin and leptin as well as influence on appetite and body weight was demonstrated.
Materials and Methods: Doses of Lipton tea (200mg/kg, 400mg/kg, and 800mg/kg) was orally administered to 25 male Wistar rats (150-200g) in various groups (n=5) respectively for 28days. Food intake, weight, visceral fats, ghrelin and leptin were assayed, and data obtained was statistically analyzed using GraphPad Prism (version 8). Statistical significance was considered at P < 0.05.
Results: Leptin, ghrelin, appetite (food intake), visceral fat mass and body weight were significantly decreased in a dose dependent manner, P < 0.05. The study suggests that the leptin level was evidence of weight reduction, while the level of ghrelin influenced the appetite which in turn affected the weight.
Conclusion: It was demonstrated that Lipton tea (a product of Camellia sinensis) induced appetite suppression via decreased ghrelin concentration, thus producing body weight and leptin reduction. This study has added to knowledge, possible mode of action of Camellia sinensis products on body weight, forming basis for its therapeutic input on weight-related pathophysiological conditions like obesity.
Keywords: Appetite Suppression; Camellia Sinensis; Lipton Tea; Ghrelin; Leptin; Weight Modulation
Increase in body weight could predispose to obesity which can be linked to risk of chronic diseases like cardiovascular diseases, chronic obstructive pulmonary diseases, diabetes etc. [1,2]. Weight modulation could help avoid the development of obesity and related pathophysiological conditions [3]. Factors like exercise and nutrition have been employed for body weight modulation [4]. Camellia sinensis and its products like black tea, green tea and Lipton have been used for its nutritional basis of regulating body weight [5]. Several studies have also reported weight reduction role of Camellia sinensis [6,7]; although its mechanism of action is not fully elucidated. It was hypothesized that the mechanism through which weight may be modulated includes appetite suppression. Ghrelin (hunger hormone), a hormone responsible for appetite stimulation and increase drive for food intake [8-10] was assayed alongside food intake, visceral fat, body weight and leptin in order to trace an aspect of weight modulation induced by Lipton tea (a product of Camellia sinensis); Hence, the study forms nutritional basis for the use of Lipton tea for weight regulation which in turn will be beneficial for obesity and associated pathophysiological conditions.
This study was part of a dissertation and performed in compliance with the principle of ethics and Guide for the Care and Use of Laboratory Animals. Twenty-five (25) male Wistar rats (150-200g) were purchased from Animal House of Gregory University Uturu, Abia State, acclimatized for two weeks and fed with grower’s marsh chow. The rats were placed in standard laboratory cages, with 24hours day/ night cycle and received humane care.
Experimental Protocol
Lipton yellow label tea (Unilever PLC, Nigeria); a product of Camellia sinensis was prepared according to method described by [5]. The experimental animals were group into 5 (n=5) and all administration were done orally (once daily) for 28 days; Group 1 (normal control) received distilled water, Group 2 (standard control) received 500 mg/kg of metformin, Group 3, 4 and 5 received 200mg/kg, 400mg/ kg and 800mg/kg of Lipton tea respectively. Furthermore, the rats were dissected on anesthesia (sodium pentobarbital 65mg/kg i.p) after 12hours of fasting on the last day of the experiment; blood samples were collected via cardiac puncture, visceral fats were harvested and put into sample bottles; samples collected were used to assay for ghrelin, leptin, and visceral fats mass.
Determination of Appetite
Appetite was determined by measuring food intake by the experimental animals. Food intake in grams/24hours were measured in the last 24 hours of administration duration using electronic top loading balance (Mettler Toledo Series) to the nearest 0.01 g. Food pellets were stored in mesh hoppers within each cage to prevent spillage and cages were visually inspected to check for loose pellets. An equal amount in grams of food was served to all groups prior to the measurement of food intake. Food intake was calculated as weight (g) of food pellets served – weight (g) of food not consumed.
Assay for Ghrelin and Leptin
4 mL of blood samples obtained were centrifuged at 10000 rpm at 4°C for 20 min; the separated serum samples were stored in an ultra- low temperature freezer at −80°C, until assay. Ghrelin levels were determined by Enzyme-Linked Immunosorbent Assay (ELISA) method using commercial kits: ghrelin ELISA kits (EMD Millipore Corporation, Billerica, MA, USA); while Leptin levels were determined using their specific Enzyme-Linked Immunosorbent Assay (ELISA) kit (Bioassay Technology, China). The protocol of the kit was strictly adhered to as was stated in the manufacturer’s manual.
Visceral Fat Mass and Body Weight Measurements
Visceral fat mass and body weight were measured as described by [5]. The absolute weights of epididymal fat, mesenteric fat and retroperitoneal fat depots were measured as intraperitoneal visceral fat mass using electronic top loading balance (Mettler Toledo Series) to the nearest 0.01 g. Body weights of the rats were also measured using electronic top loading balance (Mettler Toledo Series) to the nearest 0.01 g.
Data Analysis
GraphPad Prism (v8) was used for data analysis; multiple comparisons were done between all groups and one-way analysis of variance (ANOVA) was performed to determine statistical significance at P<0.05. Results were expressed as mean ± Standard error of mean (SEM).
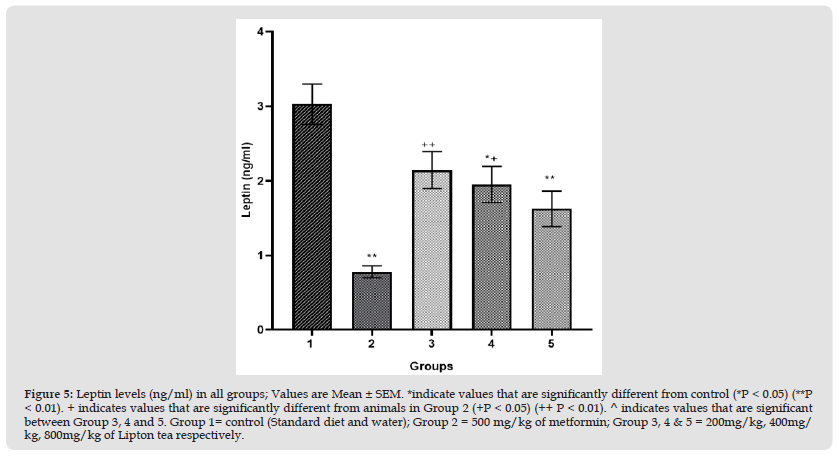
This study evaluated an aspect of weight modulation induced by Lipton tea (a product of Camellia sinensis) which involved appetite suppression. Appetite was measured as food intake in g/24hr; which was significantly decreased in a dose dependent manner on comparison between Lipton tea treated Groups (3, 4 and 5) with normal control (Group 1) at P<0.05 (Figure 1). It was observed that Lipton tea 200mg/kg, 400mg/kg and 800mg/kg decreased ghrelin (a hormone responsible for appetite stimulation) in a dose dependent manner (Figure 2) when compared with normal control at P<0.05, suggesting a decline in appetite stimulation mechanism of ghrelin. Visceral fats mass which is a contributing factor to body weight was significantly decreased in a dose dependent manner on comparison between Lipton treated Groups (3, 4 and 5) and normal control (Group 1), P<0.05 (Figure 3) which account for the significant dose dependent decrease in body weight induced by Lipton tea (Figure 4). The dose-dependent Leptin reduction effect of Lipton seen in this study (Figure 5) may be linked to the observed decrease in the body weight. Appetite suppression, weight modulation and leptin reduction effect of Lipton as seen in the study followed same trend with metformin a standard drug for weight modulation (Figures 1-5).
Figure 1 Appetite levels (Food intake in g/24hr) in all groups; Values are Mean ± SEM. *indicate values that are significantly different from control (*P < 0.05) (**P < 0.01). + indicates values that are significantly different from animals in Group 2 (+P < 0.05) (++ P < 0.01). ^ indicates values that are significant between Group 3, 4 and 5. Group 1= control (Standard diet and water); Group 2 = 500 mg/kg of metformin; Group 3, 4 & 5 = 200mg/ kg, 400mg/kg, 800mg/kg of Lipton tea respectively.
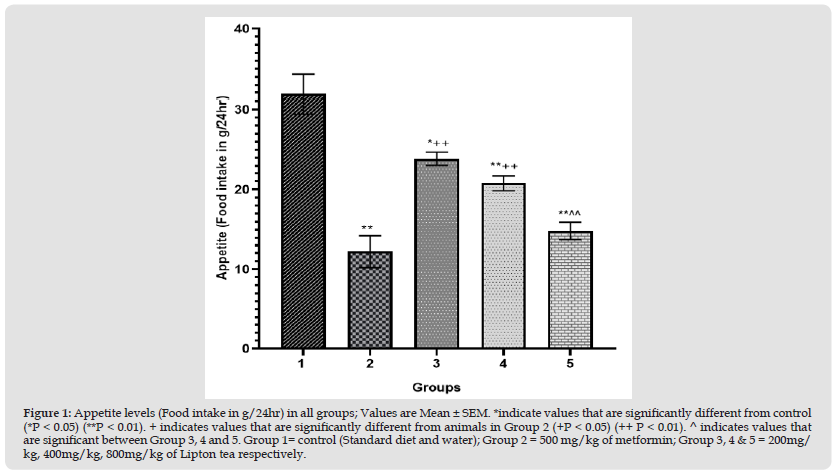
Figure 2 Ghrelin levels (pg/ml) in all groups; Values are Mean ± SEM. *indicate values that are significantly different from control (*P < 0.05) (**P < 0.01). + indicates values that are significantly different from animals in Group 2 (+P < 0.05) (++ P < 0.01). ^ indicates values that are significant between Group 3, 4 and 5. Group 1= control (Standard diet and water); Group 2 = 500 mg/kg of metformin; Group 3, 4 & 5 = 200mg/kg, 400mg/ kg, 800mg/kg of Lipton tea respectively.
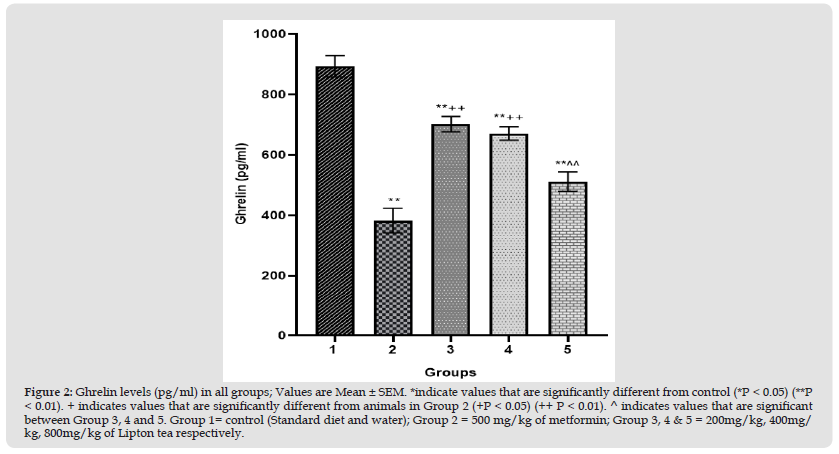
Figure 3 Visceral fat mass (g) in all groups; Values are Mean ± SEM. *indicate values that are significantly different from control (*P < 0.05) (**P < 0.01). + indicates values that are significantly different from animals in Group 2 (+P < 0.05) (++ P < 0.01). ^ indicates values that are significant between Group 3, 4 and 5. Group 1= control (Standard diet and water); Group 2 = 500 mg/kg of metformin; Group 3, 4 & 5 = 200mg/kg, 400mg/ kg, 800mg/kg of Lipton tea respectively.
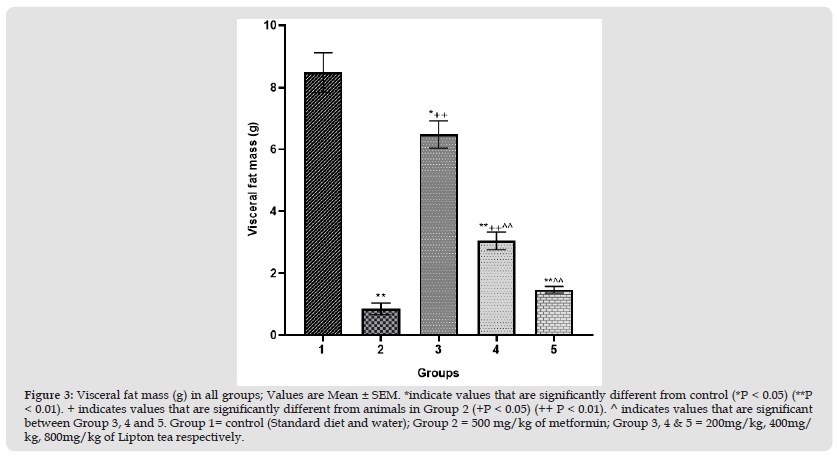
Figure 4 Body weight (g) in all groups; Values are Mean ± SEM. *indicate values that are significantly different from control (*P < 0.05) (**P < 0.01). + indicates values that are significantly different from animals in Group 2 (+P < 0.05) (++ P < 0.01). ^ indicates values that are significant between Group 3, 4 and 5. Group 1= control (Standard diet and water); Group 2 = 500 mg/kg of metformin; Group 3, 4 & 5 = 200mg/kg, 400mg/kg, 800mg/kg of Lipton tea respectively.
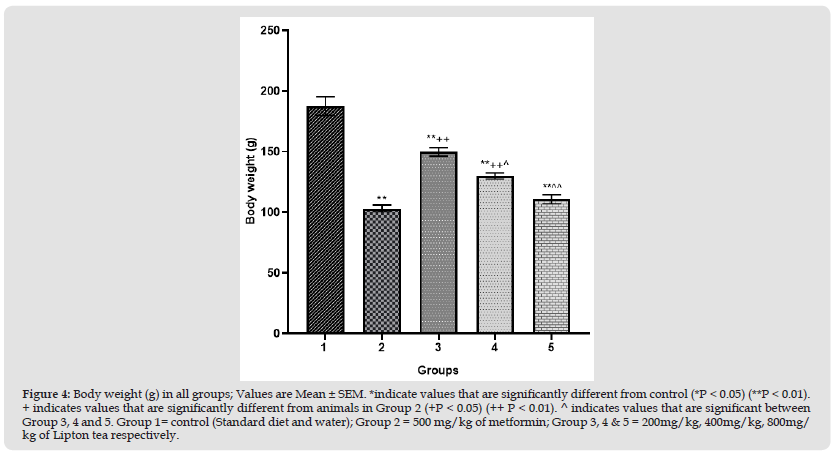
Figure 5 Leptin levels (ng/ml) in all groups; Values are Mean ± SEM. *indicate values that are significantly different from control (*P < 0.05) (**P < 0.01). + indicates values that are significantly different from animals in Group 2 (+P < 0.05) (++ P < 0.01). ^ indicates values that are significant between Group 3, 4 and 5. Group 1= control (Standard diet and water); Group 2 = 500 mg/kg of metformin; Group 3, 4 & 5 = 200mg/kg, 400mg/kg, 800mg/kg of Lipton tea respectively.
Lipton tea (a product of Camellia sinensis) has several health benefits which range from its anticancer, antioxidant and antimicrobial activities to its effectiveness in reducing body weight. It may also be required for prevention against many pathophysiological conditions, which could be traced to its chemical composition such as polyphenols, alkaloids, proteins, minerals, vitamins, amino acids, and others [11]. Considering the weight loss effect of Lipton tea reported by previous studies [5,12]; this study evaluated the role of Lipton tea on leptin, ghrelin, appetite, and visceral fat deposition in order to elucidate an aspect of Lipton tea modulatory mechanism of body weight. Lipton tea (200mg/kg, 400mg/kg and 800mg/kg) significantly decreased appetite in a dose dependent manner, which was expressed in decreased food intake (g/24hr), suggesting potent appetite suppression induced by Lipton tea, as increased appetite will increase food intake, whereas suppressed appetite will decrease food intake [13,14]. Furthermore, Ghrelin (a hunger hormone known for appetite stimulation) was significantly decreased in a dose dependent manner with response to Lipton tea administration, suggesting that the observed decline in appetite could be linked to reduction in ghrelin mechanism mediated by Lipton tea; since ghrelin is termed the ‘hunger hormone’ as it stimulates appetite, increases food intake and promotes fat storage, its decrease could impair its functions [15,16].
It was also observed that the decreased appetite suppression which resulted from reduction in ghrelin concentration mediated by Lipton tea caused weight loss as there was significant dose dependent reduction in visceral fat mass and body weight. This is because appetite influences food intake which determines the number of calories that is taken by the body and the number of calories taken influences body weight [13,14,17]. Visceral fat deposition (visceral fat depot) known to play role in protection of the internal organs as well as provides a reserve source for energy, also contributes to body weight and abnormally high deposition of visceral adipose tissue could predispose to visceral obesity [18,19]. The study also suggests that Lipton tea caused weight loss which in turn caused a decrease in leptin level. This is because leptin levels are directly connected to the amount of body fat. Increase in body fat increases leptin levels, while lower body fat percentages decrease the leptin level [20,21]. Metformin is a potent drug for weight loss including diabetes and obesity [22]. The results from this study suggest that metformin has more potent effect on weight loss than Lipton tea treated group, even as high dose of Lipton tea (800mg) showed non-significant difference in the effects. The study also suggested that Lipton followed the potent trend of metformin in reducing body weight via reduction in appetite and other parameters.
Conclusively, this study suggest that Lipton tea (a product of Camellia sinensis) decreases ghrelin level thereby causing reduction in appetite which in turn decreases visceral fat deposition and body weight; thus predisposing to leptin reduction. This study has formed the basis for Lipton tea consumption as a diet for weight regulation and can be recommended as good diet for obesity.
None.
Request@ osahmartinz@gmail.com.
Not applicable.
None.


